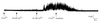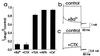hSK4, a member of a novel subfamily of calcium-activated potassium channels - PubMed (original) (raw)
hSK4, a member of a novel subfamily of calcium-activated potassium channels
W J Joiner et al. Proc Natl Acad Sci U S A. 1997.
Abstract
The gene for hSK4, a novel human small conductance calcium-activated potassium channel, or SK channel, has been identified and expressed in Chinese hamster ovary cells. In physiological saline hSK4 generates a conductance of approximately 12 pS, a value in close agreement with that of other cloned SK channels. Like other members of this family, the polypeptide encoded by hSK4 contains a previously unnoted leucine zipper-like domain in its C terminus of unknown function. hSK4 appears unique, however, in its very high affinity for Ca2+ (EC50 of 95 nM) and its predominant expression in nonexcitable tissues of adult animals. Together with the relatively low homology of hSK4 to other SK channel polypeptides (approximately 40% identical), these data suggest that hSK4 belongs to a novel subfamily of SK channels.
Figures
Figure 1
Primary structure and tissue distribution of hSK4. (a) Amino acid sequence alignment of hSK4 and rSK1 with hSK1, rSK2, and rSK3. Sequences were aligned with the computer program
pileup
(GCG) using default parameters. Gaps are represented by dots. A dark line is drawn above putative transmembrane domains, denoted by the labels S1-S6, in addition to the P region. Shading denotes absolutely conserved residues. Consensus sites for phosphorylation by protein kinases A and C are marked by open squares and circles, respectively. Leucine heptad repeats are indicated by darkened boxes. The National Center for Biotechnology Information accession numbers for the nucleotide sequences of hSK4 and rSK1 are AF000972 and AF000973, respectively. (b) Kyte–Doolittle hydrophilicity analysis of hSK4 using a window of 20 residues. Numbers along the vertical axis refer to free energy of transfer to water. (c) Dendrogram based on the alignment in a. Horizontal branch lengths are inversely proportional to the similarity between sequences, whereas vertical branch lengths are for illustrative purposes only. (d) Northern blot analysis of hSK4 transcript using 3′ untranslated cDNA as probe. Molecular sizes are indicated in kilobases.
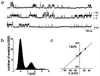
Figure 2
hSK4 generates a Ca2+-dependent, K+-selective current. (a) Whole-cell currents from a single cell patched sequentially with 0 (Top), with 907 nM (Middle), and again with 0 free Ca2+ (Bottom) in the pipette. From a holding potential of −70 mV, the membrane was given 200-ms test pulses from −140 to 80 mV in 20-mV increments. Similar results were obtained in 4/4 cells tested. (b) Current-voltage relation for the same cell as in a. (c) Sensitivity of hSK4 current to Ca2+. Whole-cell currents were measured from cells with 29, 91, 288, or 907 nM free Ca2+ in the pipette. The current density of a given cell was calculated by dividing current amplitude at 80 mV by cell capacitance. The average current density of five different cells at each Ca2+ concentration is shown. Fitting these data to the Hill equation yielded an EC50 of 95 nM Ca2+ and a Hill coefficient of 3.2. (d) Extracellular K+ shifted the membrane potential as predicted by the Nernst equation for a K+-selective channel. The membrane potential was recorded with 907 nM free Ca2+ in the pipette as bath KCl was changed from 3 to 33 mM. Each point represents the average of six cells. A linear regression through these points yields a slope of 56 mV per 10-fold change in bath K+ concentration.
Figure 3
A representative inside-out patch perfused sequentially with 0, 29 nM, and 91 nM free Ca2+ activates increasing numbers of hSK4 channels. Washout with 0 Ca2+ reverses the effect. In this and in identical experiments with other cells, patches were held at 35 mV (intracellular positive) with 130 mM K+ in the bath and 3 mM K+ in the pipette.
Figure 4
Single-channel recordings indicate hSK4 has a small conductance. (a) Discrete single-channel activity recorded continuously from a representative inside-out patch at 35 mV (intracellular positive) in the presence of 29 nM free Ca2+ in the bath. Occasional transitions to two and three openings indicate that multiple channels are present in this patch. (b) An all-points histogram of the recording in a yields two distinct peaks at 1.5 pA and 2.9 pA. (c) Single-channel current-voltage relation for the same patch, using data acquired as in b at 0, 10, and 35 mV. With 130 mM K+ in the bath and 3 mM K+ in the pipette, linear regression yields a reversal potential of −96 mV and a slope conductance of 12 pS.
Figure 5
hSK4 currents are blocked strongly by extracellular Ba2+ and charybdotoxin, weakly by TEA, and not at all by apamin or by Cs+. (a) Currents after drug treatment normalized to pretreatment levels. From a holding potential of −80 mV and with 907 nM free Ca2+ in the pipette, whole-cell currents were measured during 100–150-ms test pulses to 80 mV before and after extracellular application of 1 mM BaCl2 (Ba2+), 20 nM charybdotoxin (CTX), 5 mM TEA, 100 nM apamin (APA), or 1 mM CsCl (Cs+). For all recordings 130 mM K+ was present in the pipette, and 3 mM K+ was present in the bath. Ba2+ reduced hSK4 currents by 88 ± 3% (n = 5, P < .0005 by paired t test) and had an IC50 of 340 μM (data not shown). Charybdotoxin reduced hSK4 currents by 88 ± 4% (n = 6, P < .005 by paired t test) and had an IC50 of 2.0 nM (data not shown). TEA reduced hSK4 currents by 17 ± 3% (n = 5, P < .005 by paired t test). Apamin and Cs+ had no effect at the concentrations used here. (b) A representative recording of hSK4 currents before and after bath application of 1 mM BaCl2. In addition to blocking hSK4 currents, Ba2+ appears to alter hSK4 kinetics. (c) A representative recording of hSK4 currents before and after bath application of 20 nM charybdotoxin. Block by toxin appears to be relieved over the course of depolarizing pulses.
Similar articles
- hSK4/hIK1, a calmodulin-binding KCa channel in human T lymphocytes. Roles in proliferation and volume regulation.
Khanna R, Chang MC, Joiner WJ, Kaczmarek LK, Schlichter LC. Khanna R, et al. J Biol Chem. 1999 May 21;274(21):14838-49. doi: 10.1074/jbc.274.21.14838. J Biol Chem. 1999. PMID: 10329683 - Molecular and functional characterization of the small Ca(2+)-regulated K+ channel (rSK4) of colonic crypts.
Warth R, Hamm K, Bleich M, Kunzelmann K, von Hahn T, Schreiber R, Ullrich E, Mengel M, Trautmann N, Kindle P, Schwab A, Greger R. Warth R, et al. Pflugers Arch. 1999 Sep;438(4):437-44. doi: 10.1007/s004249900059. Pflugers Arch. 1999. PMID: 10519135 - The hSK4 (KCNN4) isoform is the Ca2+-activated K+ channel (Gardos channel) in human red blood cells.
Hoffman JF, Joiner W, Nehrke K, Potapova O, Foye K, Wickrema A. Hoffman JF, et al. Proc Natl Acad Sci U S A. 2003 Jun 10;100(12):7366-71. doi: 10.1073/pnas.1232342100. Epub 2003 May 28. Proc Natl Acad Sci U S A. 2003. PMID: 12773623 Free PMC article. - The Ca2+-activated K+ channel of intermediate conductance: a molecular target for novel treatments?
Jensen BS, Strøbaek D, Olesen SP, Christophersen P. Jensen BS, et al. Curr Drug Targets. 2001 Dec;2(4):401-22. doi: 10.2174/1389450013348173. Curr Drug Targets. 2001. PMID: 11732639 Review. - International Union of Basic and Clinical Pharmacology. C. Nomenclature and Properties of Calcium-Activated and Sodium-Activated Potassium Channels.
Kaczmarek LK, Aldrich RW, Chandy KG, Grissmer S, Wei AD, Wulff H. Kaczmarek LK, et al. Pharmacol Rev. 2017 Jan;69(1):1-11. doi: 10.1124/pr.116.012864. Epub 2016 Nov 15. Pharmacol Rev. 2017. PMID: 28267675 Free PMC article. Review.
Cited by
- SK4 Ca2+ activated K+ channel is a critical player in cardiac pacemaker derived from human embryonic stem cells.
Weisbrod D, Peretz A, Ziskind A, Menaker N, Oz S, Barad L, Eliyahu S, Itskovitz-Eldor J, Dascal N, Khananshvili D, Binah O, Attali B. Weisbrod D, et al. Proc Natl Acad Sci U S A. 2013 Apr 30;110(18):E1685-94. doi: 10.1073/pnas.1221022110. Epub 2013 Apr 15. Proc Natl Acad Sci U S A. 2013. PMID: 23589888 Free PMC article. - The slow afterhyperpolarization: a target of β1-adrenergic signaling in hippocampus-dependent memory retrieval.
Zhang L, Ouyang M, Ganellin CR, Thomas SA. Zhang L, et al. J Neurosci. 2013 Mar 13;33(11):5006-16. doi: 10.1523/JNEUROSCI.3834-12.2013. J Neurosci. 2013. PMID: 23486971 Free PMC article. - Pharmacology of Small- and Intermediate-Conductance Calcium-Activated Potassium Channels.
Brown BM, Shim H, Christophersen P, Wulff H. Brown BM, et al. Annu Rev Pharmacol Toxicol. 2020 Jan 6;60:219-240. doi: 10.1146/annurev-pharmtox-010919-023420. Epub 2019 Jul 23. Annu Rev Pharmacol Toxicol. 2020. PMID: 31337271 Free PMC article. Review. - Non-genomic regulation of intermediate conductance potassium channels by aldosterone in human colonic crypt cells.
Bowley KA, Morton MJ, Hunter M, Sandle GI. Bowley KA, et al. Gut. 2003 Jun;52(6):854-60. doi: 10.1136/gut.52.6.854. Gut. 2003. PMID: 12740342 Free PMC article.
References
- Lancaster B, Adams P R. J Neurophysiol. 1986;55:1268–1282. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous