Coupled ER to Golgi transport reconstituted with purified cytosolic proteins - PubMed (original) (raw)
Coupled ER to Golgi transport reconstituted with purified cytosolic proteins
C Barlowe. J Cell Biol. 1997.
Abstract
A cell-free vesicle fusion assay that reproduces a subreaction in transport of pro-alpha-factor from the ER to the Golgi complex has been used to fractionate yeast cytosol. Purified Sec18p, Uso1p, and LMA1 in the presence of ATP and GTP satisfies the requirement for cytosol in fusion of ER-derived vesicles with Golgi membranes. Although these purified factors are sufficient for vesicle docking and fusion, overall ER to Golgi transport in yeast semi-intact cells depends on COPII proteins (components of a membrane coat that drive vesicle budding from the ER). Thus, membrane fusion is coupled to vesicle formation in ER to Golgi transport even in the presence of saturating levels of purified fusion factors. Manipulation of the semi-intact cell assay is used to distinguish freely diffusible ER- derived vesicles containing pro-alpha-factor from docked vesicles and from fused vesicles. Uso1p mediates vesicle docking and produces a dilution resistant intermediate. Sec18p and LMA1 are not required for the docking phase, but are required for efficient fusion of ER- derived vesicles with the Golgi complex. Surprisingly, elevated levels of Sec23p complex (a subunit of the COPII coat) prevent vesicle fusion in a reversible manner, but do not interfere with vesicle docking. Ordering experiments using the dilution resistant intermediate and reversible Sec23p complex inhibition indicate Sec18p action is required before LMA1 function.
Figures
Figure 1
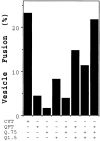
Separation of fusion factors by Mono Q anion exchange chromatography. Isolated ER-derived vesicles containing 35S-labeled gp- α-factor and acceptor membranes were incubated in a 30-μl reaction with the following protein fractions: 50 μg of cytosol (CYT), 50 μg of the Q flowthrough (QFT), 50 μg of the 0.75 M eluate (Q.75), or 15 μg of the 1.5 M eluate (Q1.5). The percent vesicle fusion represents the amount of 35S-labeled gp-α-factor that has been modified by the addition of Golgi specific α-1,6-mannose residues. In this experiment, the amount of background fusion (complete reaction minus cytosol) was 2.7%.
Figure 2
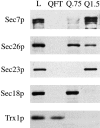
Immunoblot analysis of Mono Q fractions. Equal volumes of the load fraction (L), flow-through fraction (QFT), proteins eluting at 0.75 M KOAc (Q.75), and proteins eluting at 1.5 M KOAc (Q1.5) obtained as described under the methods section, were resolved by SDS-PAGE, followed by immunoblot for Sec7p, Sec26p, Sec23p, Sec18p, and thioredoxin (Trx1p).
Figure 3
Sec18p replaces the Q.75 fraction. (A) Vesicle fusion was measured (as in Fig. 1) with various fractions in the presence or absence of purified Sec18p-6His (50 ng) in 30 μl. (B) Vesicle fusion in the presence of QFT and Q1.5 plus increasing amounts of Sec18p-6His. In this experiment, a plus cytosol control yielded 20% vesicle fusion.
Figure 6
LMA1 replaces the QFT fraction. In A, isolated vesicles and acceptor membranes were incubated with Sec18p (50 ng) and Uso1p (75 ng) alone (column 2) or with increasing amounts of purified LMA1 (columns 3–6) in 30-μl reactions. In B, immunoblots with antithioredoxin and anti-Sec18p are shown to compare 10 ng of purified LMA1 and Sec18p-6His with 5 μg of crude cytosol (Load) and other column fractions as described in Fig. 2. The pure proteins loaded represent ∼1/5 of the amount required for saturation and the crude cytosol shown (Load) represents ∼1/10 the amount required for saturation.
Figure 6
LMA1 replaces the QFT fraction. In A, isolated vesicles and acceptor membranes were incubated with Sec18p (50 ng) and Uso1p (75 ng) alone (column 2) or with increasing amounts of purified LMA1 (columns 3–6) in 30-μl reactions. In B, immunoblots with antithioredoxin and anti-Sec18p are shown to compare 10 ng of purified LMA1 and Sec18p-6His with 5 μg of crude cytosol (Load) and other column fractions as described in Fig. 2. The pure proteins loaded represent ∼1/5 of the amount required for saturation and the crude cytosol shown (Load) represents ∼1/10 the amount required for saturation.
Figure 4
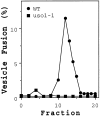
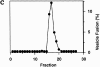
An uso1-1 strain lacks Q1.5 activity. Cytosols from a wild-type and _uso1_-1 strain were bound and eluted from a Mono Q column. Individual fractions eluting from the 0.75 to 1.5 M KOAc gradient were tested (2 μl) for stimulation of vesicle fusion in the presence of QFT and Sec18p in a 30-μl reaction volume. Data represents fusion above the QFT and Sec18p level that was 5.2% in this experiment.
Figure 5
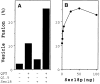
Purification of functional Uso1p-myc. Fractions eluting from a Superose 6 column were analyzed by SDS-PAGE and silver stained (A), immunoblotted with anti-myc mAb (B), and then assayed for stimulation of vesicle fusion in the presence of QFT and Sec18p-6His (C). Fraction 17 contains the peak of activity and anti-myc immunoreactivity. Activity data represents vesicle fusion above the QFT and Sec18p level that was 7% in this experiment.
Figure 5
Purification of functional Uso1p-myc. Fractions eluting from a Superose 6 column were analyzed by SDS-PAGE and silver stained (A), immunoblotted with anti-myc mAb (B), and then assayed for stimulation of vesicle fusion in the presence of QFT and Sec18p-6His (C). Fraction 17 contains the peak of activity and anti-myc immunoreactivity. Activity data represents vesicle fusion above the QFT and Sec18p level that was 7% in this experiment.
Figure 7
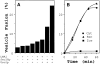
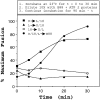
Reconstitution of vesicle fusion with purified factors. (A) Isolated, ER-derived vesicles containing 35S-labeled gp-α-factor and acceptor membranes (12 μg) were incubated with saturating amounts of individual purified factors (Sec18p [50 ng], Uso1p [75 ng], and LMA1 [50 ng]) or various combinations in 30-μl reactions. (B) Time course of vesicle fusion in the presence of purified proteins at 4°C (▪), at 23°C (▴), or with crude cytosol (50 μg) at 23°C (•).
Figure 8
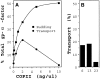
Excess COPII proteins inhibit ER to Golgi transport. (A) Budding and transport assays with semi-intact yeast cells in the presence of purified factors. Budding of 35S-labeled gp-α-factor into freely diffusible vesicles (▪) was determined in the presence of increasing amounts of COPII proteins alone (no fusion factors added). COPII concentrations reflect individual protein concentrations such that the 2 ng/μl COPII condition is 2 ng/μl Sar1p, 2 ng/μl Sec23p complex, and 2 ng/μl Sec13p complex. Transport (•) was quantified in the presence of fusion factors (Sec18p [50 ng], Uso1p [75 ng], and LMA1 [50 ng]) and varying concentrations of COPII proteins in 30-μl reactions. (B) Transport in the presence of fusion factors (as in A) and COPII proteins (2 ng/μl), plus 8 ng/μl of Sar1p (S), or 8 ng/μl Sec13p complex (13), or 8 ng/μl Sec23p complex (23).
Figure 9
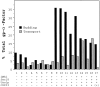
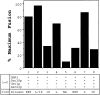
Purified fusion factors mediate distinct steps in vesicle docking and fusion. Saturating amounts of fusion factors (Sec18p [50 ng], Uso1p [75 ng], LMA1 [50 ng]) and 2 ng/μl COPII proteins were mixed in various combinations with semi-intact cells. 35S-labeled gp-α-factor contained in freely diffusible vesicles (black bars) and Golgi modified forms (hatched bars) were determined after 45 min at 23°C. The incubation on the far right (+) contains an additional 8 ng/μl of Sec23p complex.
Figure 10
Uso1p action generates a dilution resistant intermediate. Isolated vesicles were mixed on ice with acceptor membranes and individual fusion factors Uso1p (U), LMA1 (L), and Sec18p (18), or the set of fusion factors together (U/L/18). In these experiments, the concentrations of acceptor membranes, vesicles, and fusion factors were half of the standard condition (described in Fig. 7). After incubation at 23°C for various times, reactions were diluted 10-fold with one of the following: buffer containing ATP, LMA1, and Sec18p (L/18); buffer containing ATP, Uso1p, and Sec18p (U/18); buffer containing ATP, Uso1p, and LMA1 (U/L); or buffer containing ATP alone (B88). Diluents contained purified proteins at levels that produce initial concentrations of each indicated species. Each tube was incubated at the reaction temperature for a total of 90 min. In this experiment, background fusion (vesicles, acceptor membranes, and ATP, undiluted) was 1.7% and maximal fusion (vesicles, acceptor membranes, fusion factors, and ATP, undiluted) was 12%.
Figure 11
Sec18p is required before LMA1 action. Reaction conditions are as described in Fig. 10 except Sec23p (6 ng/μl) was added to indicated reactions. After a 20-min incubation at 23°C, reactions were left undiluted (NA) or diluted 10-fold with buffer containing ATP (B88), buffer containing ATP and Sec18p (18), buffer containing ATP and LMA (L), or buffer containing ATP, LMA, and Sec18p (L/18). Each tube was incubated at the reaction temperature for a total of 90 min. In this experiment, background fusion was 2.3% and maximal fusion was 19%.
Similar articles
- Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins.
Cao X, Ballew N, Barlowe C. Cao X, et al. EMBO J. 1998 Apr 15;17(8):2156-65. doi: 10.1093/emboj/17.8.2156. EMBO J. 1998. PMID: 9545229 Free PMC article. - Reconstitution of retrograde transport from the Golgi to the ER in vitro.
Spang A, Schekman R. Spang A, et al. J Cell Biol. 1998 Nov 2;143(3):589-99. doi: 10.1083/jcb.143.3.589. J Cell Biol. 1998. PMID: 9813082 Free PMC article. - Erv25p, a component of COPII-coated vesicles, forms a complex with Emp24p that is required for efficient endoplasmic reticulum to Golgi transport.
Belden WJ, Barlowe C. Belden WJ, et al. J Biol Chem. 1996 Oct 25;271(43):26939-46. doi: 10.1074/jbc.271.43.26939. J Biol Chem. 1996. PMID: 8900179 - Exiting the endoplasmic reticulum.
Gorelick FS, Shugrue C. Gorelick FS, et al. Mol Cell Endocrinol. 2001 May 25;177(1-2):13-8. doi: 10.1016/s0303-7207(01)00438-5. Mol Cell Endocrinol. 2001. PMID: 11377815 Review. - COPII: a membrane coat that forms endoplasmic reticulum-derived vesicles.
Barlowe C. Barlowe C. FEBS Lett. 1995 Aug 1;369(1):93-6. doi: 10.1016/0014-5793(95)00618-j. FEBS Lett. 1995. PMID: 7641893 Review.
Cited by
- mBet3p is required for homotypic COPII vesicle tethering in mammalian cells.
Yu S, Satoh A, Pypaert M, Mullen K, Hay JC, Ferro-Novick S. Yu S, et al. J Cell Biol. 2006 Jul 31;174(3):359-68. doi: 10.1083/jcb.200603044. J Cell Biol. 2006. PMID: 16880271 Free PMC article. - Asymmetric requirements for a Rab GTPase and SNARE proteins in fusion of COPII vesicles with acceptor membranes.
Cao X, Barlowe C. Cao X, et al. J Cell Biol. 2000 Apr 3;149(1):55-66. doi: 10.1083/jcb.149.1.55. J Cell Biol. 2000. PMID: 10747087 Free PMC article. - The redox sensor TXNL1 plays a regulatory role in fluid phase endocytosis.
Felberbaum-Corti M, Morel E, Cavalli V, Vilbois F, Gruenberg J. Felberbaum-Corti M, et al. PLoS One. 2007 Nov 7;2(11):e1144. doi: 10.1371/journal.pone.0001144. PLoS One. 2007. PMID: 17987124 Free PMC article. - ER to Golgi transport: Requirement for p115 at a pre-Golgi VTC stage.
Alvarez C, Fujita H, Hubbard A, Sztul E. Alvarez C, et al. J Cell Biol. 1999 Dec 13;147(6):1205-22. doi: 10.1083/jcb.147.6.1205. J Cell Biol. 1999. PMID: 10601335 Free PMC article. - New links between vesicle coats and Rab-mediated vesicle targeting.
Angers CG, Merz AJ. Angers CG, et al. Semin Cell Dev Biol. 2011 Feb;22(1):18-26. doi: 10.1016/j.semcdb.2010.07.003. Epub 2010 Jul 17. Semin Cell Dev Biol. 2011. PMID: 20643221 Free PMC article. Review.
References
- Baker D, Hicke L, Rexach M, Schleyer M, Schekman R. Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell. 1988;54:335–344. - PubMed
- Ballou C. A study of the immunochemistry of three yeast mannans. J Biol Chem. 1970;245:1197–1203. - PubMed
- Barlowe C, Orci L, Yeung T, Hosobuchi D, Hamamoto S, Salama N, Rexach M, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the ER. Cell. 1994;77:895–907. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases