Accumulation of muscle ankyrin repeat protein transcript reveals local activation of primary myotube endcompartments during muscle morphogenesis - PubMed (original) (raw)
Accumulation of muscle ankyrin repeat protein transcript reveals local activation of primary myotube endcompartments during muscle morphogenesis
A Baumeister et al. J Cell Biol. 1997.
Abstract
The characteristic shapes and positions of each individual body muscle are established during the process of muscle morphogenesis in response to patterning information from the surrounding mesenchyme. Throughout muscle morphogenesis, primary myotubes are arranged in small parallel bundles, each myotube spanning the forming muscles from end to end. This unique arrangement potentially assigns a crucial role to primary myotube end regions for muscle morphogenesis. We have cloned muscle ankyrin repeat protein (MARP) as a gene induced in adult rat skeletal muscle by denervation. MARP is the rodent homologue of human C-193 (Chu, W., D.K. Burns, R.A. Swerick, and D.H. Presky. 1995. J. Biol. Chem. 270:10236-10245) and is identical to rat cardiac ankyrin repeat protein. (Zou, Y., S. Evans, J. Chen, H.-C. Kuo, R.P. Harvey, and K.R. Chien. 1997. Development. 124:793-804). In denervated muscle fibers, MARP transcript accumulated in a unique perisynaptic pattern. MARP was also expressed in large blood vessels and in cardiac muscle, where it was further induced by cardiac hypertrophy. During embryonic development, MARP was expressed in forming skeletal muscle. In situ hybridization analysis in mouse embryos revealed that MARP transcript exclusively accumulates at the end regions of primary myotubes during muscle morphogenesis. This closely coincided with the expression of thrombospondin-4 in adjacent prospective tendon mesenchyme, suggesting that these two compartments may constitute a functional unit involved in muscle morphogenesis. Transfection experiments established that MARP protein accumulates in the nucleus and that the levels of both MARP mRNA and protein are controlled by rapid degradation mechanisms characteristic of regulatory early response genes. The results establish the existence of novel regulatory muscle fiber subcompartments associated with muscle morphogenesis and denervation and suggest that MARP may be a crucial nuclear cofactor in local signaling pathways from prospective tendon mesenchyme to forming muscle and from activated muscle interstitial cells to denervated muscle fibers.
Figures
Figure 1
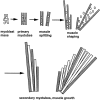
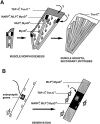
Schematic representation of the spatial arrangement of myogenic cells during muscle formation. Muscle morphogenesis produces the definitive shape and arrangement of each individual muscle and precedes the formation of secondary myotubes. From their first appearance, primary myotubes (shaded rectangles) span forming muscles in a parallel end-to-end arrangement. During muscle morphogenesis, a substantial part of the muscle mass contains undifferentiated myoblasts (circles), and primary myotubes are arranged in parallel bundles of three to seven. When morphogenesis is completed, primary myotube bundles dissociate, and single primary myotubes serve as scaffolds along which secondary myotubes (white rectangles) fuse and elongate. Note that due to the particular arrangement of the primary myotubes, muscle splitting and shaping may in principle be regulated by controlling the position and local elongation of primary myotube end regions. The latter process may involve selective fusion of myoblasts to primary myotube end regions.
Figure 2
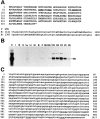
Sequence of rat MARP and of the mouse MARP promoter. Induction of MARP in denervated skeletal muscle. (A) Deduced amino acid sequence of rat MARP. The bipartite nuclear localization sequence is underlined, and the first residues of the four ankyrin repeats are in bold. (3′ UTR) Partial sequences of rat (R) and human (H) MARP mRNA with the ATTTA degradation sequences (bold). (B) MARP mRNA contents in adult rat tissues. Northern blot of comparable amounts of total RNA from heart (H), intestine (I), diaphragm muscle (M), skin (S), lung (Lu), liver (Li), kidney (Ki), brain (B), placenta (P), and gastrocnemius muscle before (D0) and 0.7, 2, 3, and 7 d after denervation, or 3 and 8 d after Botulinum toxin-A–induced paralysis (P). Note strong induction by denervation or paralysis of skeletal muscle. (C) Nucleotide sequence of the proximal promoter of mouse MARP. Muscle E-box sequences are in bold; muscle CCAC boxes are underlined.
Figure 3
Local patterns of MARP transcript induction in adult denervated muscle and hypertrophic heart. (A) Perisynaptic accumulation of MARP transcript in denervated muscle. Gastrocnemius muscle of adult mice was collected 7 d after resection of the sciatic nerve. Note prominent MARP in situ hybridization signal at a broad region near to acetylcholine esterase reaction product (adjacent section on the right). As expected from the absence of the corresponding mRNA on Northern blots, no MARP signal was detected in control muscle (right). (B) High-magnification photographs of MARP (left) and TSP-4 (right) transcript localization in the perisynaptic region of 7-d denervated gastrocnemius muscle. Note MARP signal along the inner face of striated muscle fibers. Distribution of the TSP-4 transcript, which accumulates in a muscle interstitial cell pattern clearly distinct from that of MARP is shown for comparison. (C) Induction of MARP transcript in the hypertrophic heart. (Left) In situ hybridization for MARP mRNA in adult mouse heart. In control heart MARP mRNA is expressed at high levels in atria and at low levels in ventricles. In the hearts of MLP-deficient mice (−/−) with dilated cardiomyopathy and hypertrophy (Arber et al., 1997), MARP transcript is strongly upregulated in ventricles. (Right) Selective upregulation of MARP mRNA in hypertrophic ventricles. The Northern blot was hybridized with a MARP probe and comparable amounts of total RNA from adult ventricle (H) or gastrocnemius muscle (Mu) from control or MLP-deficient (−/−) mice were applied to the gel. Bar: (A) 70 μm, (B, MARP) 8 μm, (B, TSP-4) 16 μm.
Figure 4
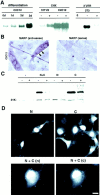
MARP has properties of a nuclear early response gene. (A) Regulation of MARP mRNA contents by myogenic differentiation, cycloheximide, and 3′ untranslated sequences (UTR). The RNA blots (equivalent amounts of total RNA for each blot) were hybridized with a rat MARP probe. The experiment on the left was carried out with a C2C12 subclone exhibiting MARP induction during differentiation (days). The C2C12 subclone used for the cycloheximide (CHX) induction experiment already expressed high levels of MARP in high-serum medium, whereas CHX induced MARP transcript in 10T1/2 fibroblasts. (− and +) RNA collected after 6 h without or with CHX. For the experiments on the right, Swiss 3T3 fibroblasts were transfected with MARP expression constructs containing (+) or devoid (−) of 3′ untranslated region sequences, and RNA was collected 2 d after transfection. The presence of MARP 3′ untranslated region sequences led to greatly reduced transcript levels. (0) Nontransfected cells. (B) In differentiating C2C12 cells MARP transcript selectively accumulates in multinucleated myotubes (arrows). In situ hybridization of cultures 3 d after switch to differentiation medium. (Right) MARP sense probe as a negative control. The C2C12 subclone was as in (A) (Left) Note homogeneous pattern of MARP transcript accumulation in myotubes in vitro. (C) MARP protein is rapidly degraded in transfected cells, and degradation can be delayed by the fusion of short peptide sequences to the carboxyl-terminal end of the protein. COS cells were transfected with MARP expression constructs, and MARP contents were determined on the immunoblot (anti-MARP antiserum). Equal amounts of cell homogenate protein were applied for each sample (see background band at ∼31 kD). (N + C) myc-tag at the amino terminus and GLVVMNIT-tag at the carboxy terminus; (N) myc-tag at the amino terminus; (C) myc-tag at the carboxyl terminus; (−) no epitope tags. For each MARP construct, cells were incubated with (+) or without (−) CHX during the last 8 h before homogenization. (D) Nuclear and cytosolic accumulation of MARP in transfected COS cells. The epitope-tagged constructs are defined as described in (C). (Top) Anti-myc labeling of amino- (left) and carboxyl-terminal–tagged (right) MARP. Note higher frequency of labeled cells and of cytosolic signal in the presence of carboxyl-terminal tag sequence. Double-tagged (N + C) MARP was visualized by double labeling for the amino terminus (n) and carboxyl terminus (c) epitope tag. Note nuclear accumulation of undegraded MARP. Bar: (B) 15 μm; (D, single labelings) 9 μm; (D, double labeling) 6 μm.
Figure 5
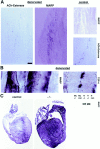
During muscle formation in vivo, appearance of MARP transcript coincides with terminal differentiation, and the transcript selectively accumulates at muscle end regions. In situ hybridization of mouse embryo sections. MyoD is expressed in myogenic cells before and after terminal differentiation; MLP is a marker for terminally differentiated striated muscle cells. The horizontal lines indicate consecutive sections (14 μm). (A) At E11.5, MARP mRNA is detectable within MLP-positive myotome (left), whereas in the forelimb, MyoD-positive myogenic progenitor cells were negative for MLP and MARP transcripts. (B) At E14.5, MARP transcript accumulated at end regions of MLP- and MyoD-positive muscles (top; the example is from foot muscles). A detail from the top panels (arrows) is shown in the middle panels. Note restriction of MARP transcript to edge of the indentation in the MLP-positive muscle. The bottom panels show an example of MARP transcript accumulation at the end of a MyoD-positive muscle (between ribs). In the corresponding higher magnification photograph on the right, MARP transcript can be detected inside striated cells, i.e., primary myotubes (arrows point to spared nuclei). Bar: (A) 480 μm; (B, top) 240 μm; (B, middle) 30 μm; (B, bottom, left) 60 μm; (B, bottom, right) 15 μm.
Figure 7
Accumulation of MARP transcript at end regions of skeletal muscle during muscle morphogenesis. In situ hybridization of consecutive sections labeled for MARP (left) and MLP (right) transcript. The sections display different groups of muscles between E12.5 and 17.5 (E12.5, face; E13.5 and 15.5, hindpaw; E17.5, front paw). Note consistent restriction of MARP transcript to myotube end regions. MARP expression was strongly downregulated at E17.5. Bar: (E12.5) 180 μm; (E13.5, 15.5, and 17.5) 360 μm.
Figure 6
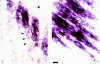
Accumulation of MARP transcript at end regions of primary myotubes. Two high-magnification examples of MARP transcript localization at end regions of facial muscles in an E14.5 mouse embryo are shown. (Left) Nonmuscle region (top right) is marked with an asterisk, and the three arrows delineate the nonlabeled part of a primary myotube bundle (striations) that extends towards the opposite end of the muscle; MARP signal is restricted to the end regions of the primary myotube bundles. (Right) In this example the muscle end is at the top left corner; multinucleated (arrows; nuclei are spared in the in situ hybridization labeling) primary myotube bundles are labeled from the muscle end, and signal intensity decreases towards the lower right corner of the figure, i.e., towards the opposite end of the muscle. Bar, 11 μm.
Figure 8
At muscle end regions, MARP and TSP-4 transcripts accumulate at adjacent locations. Consecutive sections (chest muscles, above heart) from an E14.5 embryo were hybridized for MLP, MARP, and TSP-4 transcript, as indicated. Note similar distribution and complementarity of MARP and TSP-4 labeling patterns (two examples are indicated by the arrowheads). In addition to regions adjacent to muscle ends, TSP-4 transcript also accumulated in chondrogenic areas (one example indicated by the asterisk). Bar, 500 μm.
Figure 9
TSP-4 transcript accumulates at prospective tendon mesenchyme regions adjacent to MARP-positive primary myotube ends. In situ hybridization experiments; horizontal lines indicate adjacent sections. (A) Spatial and temporal correlation of MARP and TSP-4 transcript accumulation. Trunk muscles, cervical region. Note TSP-4 signal adjacent to MARP (e.g., at muscle indentations). Also note that at E17.5 both MARP and TSP-4 signal were downregulated. (B) MARP transcript accumulates at the ends of primary myotubes, adjacent to TSP-4–expressing prospective tendon mesenchyme. Consecutive sections from E13.5 (top) and E15.5 (bottom) intercostal muscle. Note coextensive arrangement of MARP- and TSP-4–expressing structures. H&E, Haematoxilin and eosin stain. Bar: (A) 1.1 mm, (B, top) 35 μm, (B, bottom) 140 μm.
Figure 10
Myogenic differentiation in the presence of COS-TSP-4 cells. Cocultures of control (top; COS) or TSP-4–expressing (bottom; COS-TSP-4) COS cells with newborn hind limb muscle- derived cells. Culture dishes were precoated with laminin, and experimental details were as described in Materials and Methods. The phase contrast photographs of living cells were taken 1 (left) and 2 (right) d after switch to differentiation medium. The photographs on the right show immunocytochemistry of muscle-specific α actinin 2 d after induction of differentiation. Note that in the presence of naive COS cells, muscle-derived cells frequently formed chains (top, center) of cells that expressed low levels of sarcomeric α actinin (top, right) but in most cases failed to fuse to myotubes. In contrast, efficient formation of myotubes that expressed high levels of sarcomeric α actinin was detected in the presence of COS-TSP-4 cells (bottom). Bar: (Left and middle; phase-contrast) 24 μm; (right; immunofluorescence) 12 μm.
Figure 11
The local accumulation of MARP mRNA visualizes novel regulatory muscle subcompartments during muscle morphogenesis and in denervated muscle. (A) During muscle morphogenesis, MARP mRNA accumulates at primary myotube end regions, adjacent to TSP-4–expressing prospective tendon mesenchyme. In contrast, MLP transcript accumulates throughout primary myotubes, and that for MyoD is also detected in myoblasts. The arrows with question marks indicate the possible direction of signaling, from surrounding mesenchyme to tendon mesenchyme to primary myotube end regions. During muscle morphogenesis, extensive myoblast proliferation, fusion to preexisting primary myotubes, and myotube elongation may be restricted to muscle end regions under the control of prospective tendon mesenchyme. (B) In denervated adult skeletal muscle, MARP mRNA accumulates in the perisynaptic region of muscle fibers, in the vicinity of activated muscle interstitial cells. The arrow with question mark indicates the possible direction of signaling from activated muscle interstitial cells to the perisynaptic region of denervated muscle. Unlike MARP mRNA, transcripts for classical denervation- induced genes such as MyoD and MLP accumulate throughout denervated muscle fibers. In addition, subsynaptic muscle nuclei express distinct genes in innervated and denervated muscle.
Similar articles
- Multifunctional protein: cardiac ankyrin repeat protein.
Zhang N, Xie XJ, Wang JA. Zhang N, et al. J Zhejiang Univ Sci B. 2016 May;17(5):333-41. doi: 10.1631/jzus.B1500247. J Zhejiang Univ Sci B. 2016. PMID: 27143260 Free PMC article. Review. - A novel cardiac-restricted target for doxorubicin. CARP, a nuclear modulator of gene expression in cardiac progenitor cells and cardiomyocytes.
Jeyaseelan R, Poizat C, Baker RK, Abdishoo S, Isterabadi LB, Lyons GE, Kedes L. Jeyaseelan R, et al. J Biol Chem. 1997 Sep 5;272(36):22800-8. doi: 10.1074/jbc.272.36.22800. J Biol Chem. 1997. PMID: 9278441 - Structural and regulatory roles of muscle ankyrin repeat protein family in skeletal muscle.
Barash IA, Bang ML, Mathew L, Greaser ML, Chen J, Lieber RL. Barash IA, et al. Am J Physiol Cell Physiol. 2007 Jul;293(1):C218-27. doi: 10.1152/ajpcell.00055.2007. Epub 2007 Mar 28. Am J Physiol Cell Physiol. 2007. PMID: 17392382 - The enigmatic role of the ankyrin repeat domain 1 gene in heart development and disease.
Mikhailov AT, Torrado M. Mikhailov AT, et al. Int J Dev Biol. 2008;52(7):811-21. doi: 10.1387/ijdb.082655am. Int J Dev Biol. 2008. PMID: 18956313 Review.
Cited by
- CARP, a cardiac ankyrin repeat protein, is up-regulated during wound healing and induces angiogenesis in experimental granulation tissue.
Shi Y, Reitmaier B, Regenbogen J, Slowey RM, Opalenik SR, Wolf E, Goppelt A, Davidson JM. Shi Y, et al. Am J Pathol. 2005 Jan;166(1):303-12. doi: 10.1016/S0002-9440(10)62254-7. Am J Pathol. 2005. PMID: 15632022 Free PMC article. - Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth, and anatomical plasticity.
Frey D, Laux T, Xu L, Schneider C, Caroni P. Frey D, et al. J Cell Biol. 2000 Jun 26;149(7):1443-54. doi: 10.1083/jcb.149.7.1443. J Cell Biol. 2000. PMID: 10871284 Free PMC article. - Single-nucleus RNA-seq identifies transcriptional heterogeneity in multinucleated skeletal myofibers.
Petrany MJ, Swoboda CO, Sun C, Chetal K, Chen X, Weirauch MT, Salomonis N, Millay DP. Petrany MJ, et al. Nat Commun. 2020 Dec 11;11(1):6374. doi: 10.1038/s41467-020-20063-w. Nat Commun. 2020. PMID: 33311464 Free PMC article. - Research progress of ankyrin repeat domain 1 protein: an updated review.
Xu X, Wang X, Li Y, Chen R, Wen H, Wang Y, Ma G. Xu X, et al. Cell Mol Biol Lett. 2024 Oct 17;29(1):131. doi: 10.1186/s11658-024-00647-w. Cell Mol Biol Lett. 2024. PMID: 39420247 Free PMC article. Review. - Multifunctional protein: cardiac ankyrin repeat protein.
Zhang N, Xie XJ, Wang JA. Zhang N, et al. J Zhejiang Univ Sci B. 2016 May;17(5):333-41. doi: 10.1631/jzus.B1500247. J Zhejiang Univ Sci B. 2016. PMID: 27143260 Free PMC article. Review.
References
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner H-R, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. - PubMed
- Arber S, Halder G, Caroni P. Muscle LIM protein, a novel positive regulator of myogenesis, promotes myogenic differentiation. Cell. 1994;79:221–231. - PubMed
- Arber S, Hunter JJ, Ross J, Jr, Hongo M, Sansig G, Borg J, Perriard J-C, Chien KR, Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1997;88:393–403. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases