Regulation of beta-catenin levels and localization by overexpression of plakoglobin and inhibition of the ubiquitin-proteasome system - PubMed (original) (raw)
Regulation of beta-catenin levels and localization by overexpression of plakoglobin and inhibition of the ubiquitin-proteasome system
D Salomon et al. J Cell Biol. 1997.
Abstract
beta-Catenin and plakoglobin (gamma-catenin) are closely related molecules of the armadillo family of proteins. They are localized at the submembrane plaques of cell-cell adherens junctions where they form independent complexes with classical cadherins and alpha-catenin to establish the link with the actin cytoskeleton. Plakoglobin is also found in a complex with desmosomal cadherins and is involved in anchoring intermediate filaments to desmosomal plaques. In addition to their role in junctional assembly, beta-catenin has been shown to play an essential role in signal transduction by the Wnt pathway that results in its translocation into the nucleus. To study the relationship between plakoglobin expression and the level of beta-catenin, and the localization of these proteins in the same cell, we employed two different tumor cell lines that express N-cadherin, and alpha- and beta-catenin, but no plakoglobin or desmosomal components. Individual clones expressing various levels of plakoglobin were established by stable transfection. Plakoglobin overexpression resulted in a dose-dependent decrease in the level of beta-catenin in each clone. Induction of plakoglobin expression increased the turnover of beta-catenin without affecting RNA levels, suggesting posttranslational regulation of beta-catenin. In plakoglobin overexpressing cells, both beta-catenin and plakoglobin were localized at cell-cell junctions. Stable transfection of mutant plakoglobin molecules showed that deletion of the N-cadherin binding domain, but not the alpha-catenin binding domain, abolished beta-catenin downregulation. Inhibition of the ubiquitin-proteasome pathway in plakoglobin overexpressing cells blocked the decrease in beta-catenin levels and resulted in accumulation of both beta-catenin and plakoglobin in the nucleus. These results suggest that (a) plakoglobin substitutes effectively with beta-catenin for association with N-cadherin in adherens junctions, (b) extrajunctional beta-catenin is rapidly degraded by the proteasome-ubiquitin system but, (c) excess beta-catenin and plakoglobin translocate into the nucleus.
Figures
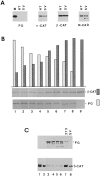
Figure 1
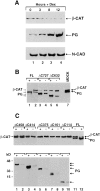
Expression of β-catenin in HT1080 and SVT2 cells overexpressing plakoglobin. (A) Equal amounts of total cell protein from HT1080 (HT), SVT2 (SV), and MDCK (M) cells were analyzed by immunoblotting with antibodies against plakoglobin (PG), α-catenin (_α_-CAT), β-catenin (_β_-CAT), and N-cadherin (N-CAD). (B) HT1080 cells were transfected with plakoglobin cDNA and equal amounts of total cell protein from individual clones stably expressing varying levels of plakoglobin and (cultured in the presence of dexamethasone) were analyzed by immunoblotting with antibodies against plakoglobin and β-catenin. The bars represent densitometer tracing of the intensity of plakoglobin (dotted bars) and β-catenin (filled bars) bands. C, SVT2 cells were stably transfected with plakoglobin and clones expressing varying levels of the transgene (lanes 1–6) were analyzed as in B. Lane 7, nontransfected 3T3 cells; lane 8, control SVT2 cells.
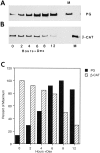
Figure 2
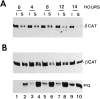
Regulation of β-catenin levels in HT1080 cells stably transfected with plakoglobin under an inducible promoter. (A) HT1080 cells expressing plakoglobin under a dexamethasone- inducible MMTV promoter were stimulated with dexamethasone, and at different time points after stimulation equal amounts of total cell protein were analyzed for plakoglobin expression by immunoblotting with antibodies against plakoglobin (PG). (B) Simultaneous analysis, on the same protein blot, of β-catenin (_β_-CAT) expression at different times after induction with dexamethasone. (C) Densitometer tracing of the levels of plakoglobin (filled bars) and β-catenin (hatched bars) expressed in A and B. M, MDCK cell lysates.
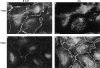
Figure 3
Organization of β-catenin and plakoglobin in HT1080 cells before and after induction of plakoglobin expression. HT1080 cells expressing inducible plakoglobin (as described in Fig. 2) were doubly stained with polyclonal anti–β-catenin (A and C) and monoclonal anti-plakoglobin (B and D) antibodies, before (A and B) and 12 h after (C and D) induction with dexamethasone (Dex). The secondary antibodies were FITC–anti-rabbit antibody and rhodamine anti–mouse-IgG. Bar, 10 μm.
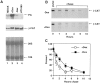
Figure 4
Expression of β-catenin RNA and stability of β-catenin protein in cells overexpressing inducible plakoglobin. (A) RNA was extracted from MDCK cells (lane 1), untransfected HT1080 cells (lane 4), and plakoglobin-transfected HT1080 cells before (lane 3), and 12 h after stimulation with dexamethasone (lane 2). Equal amounts of total cell RNA were analyzed by Northern blot hybridization with plakoglobin (PG) and β-catenin (β_-CAT) cDNAs. The levels of 18 and 28S rRNA are shown for comparison. (B) Uninduced (−_Dex) and cells induced for 12 h (+Dex) were pulse labeled with [35S]methionine for 30 min, followed by chase with fresh medium. Equal amounts of radioactive cellular proteins were immunoprecipitated with anti–β-catenin antibody and analyzed by SDS-PAGE. (C) The radioactivity in the β-catenin band in B and in an identical independent experiment was determined by a phosphorimager, and the values ±SD are presented as percent of the values obtained after 30 min pulse labeling.
Figure 5
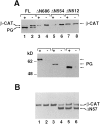
β-Catenin and plakoglobin levels in complexes with _N_-cadherin in cells transfected with COOH-terminal deletion mutant plakoglobin under control of an inducible promoter. (A) Equal amounts of cellular protein from unstimulated HT1080 cells (lane 1) and cells induced with dexamethasone to express plakoglobin driven by the MMTV promoter for: 3 (lane 2), 8 (lane 3) and 12 h (lane 4) were immunoprecipitated with anti– _N_-cadherin antibody and the protein blot was reacted with anti β-catenin (_β_-CAT), plakoglobin (PG), and anti–N-cadherin (N-CAD) antibodies. (B) Cells expressing full-length (FL), or COOH-terminal–truncated (ΔC) plakoglobin retaining 727 (ΔC727) and 632 (ΔC632) amino acids. (C) Cells expressing COOH-terminal plakoglobin deletions containing 458 (Δ458), 414 (Δ414), 375 (Δ375), 161 (Δ161), and 114 (Δ114) amino acids. Uninduced (−) and cells induced (+) with dexamethasone, to express the different plakoglobin constructs, were analyzed by immunoblotting with anti-plakoglobin (PG) and β-catenin (_β_-CAT) antibodies on the same blots.
Figure 9
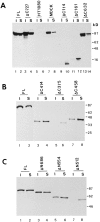
Schematic representation of plakoglobin functional domains involved in reducing β-catenin levels and in complexing with α-catenin and N-cadherin. Full-length human plakoglobin (745 amino acids) is shown with the 13 armadillo repeats and the different deletion mutants from the NH2 (ΔN) and COOH terminus (ΔC) used in this study. The binding studies to α-catenin and N-cadherin of the various plakoglobin mutants were described (Sacco et al., 1995; Wahl et al., 1996). The binding sites for the monoclonal antibodies 11E4 and PG 5.1 used in this study are also indicated.
Figure 6
Levels of β-catenin in cells expressing inducible NH2-terminal deleted plakoglobin and β-catenin mutants. (A) Levels of β-catenin in cells expressing dexamethasone-inducible plakoglobin mutants with NH2-terminal deletions retaining 686 (ΔN686), 554 (ΔN554), or 512 (ΔN512) amino acid, were determined as in Fig. 5. (B) Levels of β-catenin in individual clones stably expressing an NH2-terminal deleted β-catenin lacking the first 57 amino acids (ΔN57, lanes 4–6) or neor controls (lanes 1–3).
Figure 7
The solubility in Triton X-100 of full-length and plakoglobin mutants. (A) HT1080 cells expressing full-length plakoglobin (FL), untransfected HT1080, MDCK, and cells expressing COOH-terminal deletions of plakoglobin containing 727 (Δ727), 632 (Δ632), 161 (Δ161), and 114 (Δ114) amino acids were separated into Triton X-100–soluble (S) and –insoluble (I) fractions. Equal volumes of each fraction were analyzed for plakoglobin levels by immunoblotting. (B) HT1080 expressing COOH-terminal deletions that contain 458 (Δ458), 414 (Δ414), and 375 (Δ375) amino acids were analyzed as described in A. (C) Analysis of the Triton X-100 solubility of NH2-terminal plakoglobin deletions containing 686 (Δ686), 554 (Δ554), and 512 (Δ512) amino acids. In A and B the 11E4 antibody was used, while in C antibody PG 5.1 was used (see Fig. 9 for the plakoglobin domains recognized by these antibodies).
Figure 8
Triton X-100 solubility of β-catenin in cells expressing full-length and mutant plakoglobin. HT1080 cells transfected with full-length plakoglobin (A), or with the COOH-terminal deletion mutant ΔC161 (B) were stimulated to express plakoglobin by dexamethasone, and at various times after induction the levels of plakoglobin and β-catenin were determined in the Triton X-100– soluble and –insoluble fractions as described in Fig. 7.
Figure 10
Expression of β-catenin and plakoglobin in cells treated with inhibitors of the ubiquitin-proteasome system. (A) HT1080 cells were treated for 2 h with proteasome inhibitors (MG 132 or ALLN), or with the inactive analogue ALLM, as described in Materials and Methods, and then induced to express plakoglobin with dexamethasone in the presence of the inhibitors. At different times after dexamethasone stimulation, equal amounts of total cell lysate were analyzed for β-catenin and plakoglobin expression by immunoblotting as described in Fig. 8. (B) The immunoblot for β-catenin was overexposed to reveal the higher molecular mass forms of β-catenin (arrowhead, probably ubiquitinated) formed in the presence of the proteasome inhibitors.
Figure 11
The localization of β-catenin and plakoglobin in cells treated with inhibitors of the ubiquitin-proteasome system. HT1080 cells were pretreated with the inactive analogue ALLM (A), with the proteasome inhibitors ALLN (B and D), or with MG-132 in the presence of dexamethasone to induce plakoglobin expression (C). After 9 h, the cells were immunostained for β-catenin (A–C) or plakoglobin (D). Bar, 10 μm.
Similar articles
- Differential interaction of plakoglobin and beta-catenin with the ubiquitin-proteasome system.
Sadot E, Simcha I, Iwai K, Ciechanover A, Geiger B, Ben-Ze'ev A. Sadot E, et al. Oncogene. 2000 Apr 13;19(16):1992-2001. doi: 10.1038/sj.onc.1203519. Oncogene. 2000. PMID: 10803460 - Differential nuclear translocation and transactivation potential of beta-catenin and plakoglobin.
Simcha I, Shtutman M, Salomon D, Zhurinsky J, Sadot E, Geiger B, Ben-Ze'ev A. Simcha I, et al. J Cell Biol. 1998 Jun 15;141(6):1433-48. doi: 10.1083/jcb.141.6.1433. J Cell Biol. 1998. PMID: 9628899 Free PMC article. - Tyrosine phosphorylation of plakoglobin causes contrary effects on its association with desmosomes and adherens junction components and modulates beta-catenin-mediated transcription.
Miravet S, Piedra J, Castaño J, Raurell I, Francí C, Duñach M, García de Herreros A. Miravet S, et al. Mol Cell Biol. 2003 Oct;23(20):7391-402. doi: 10.1128/MCB.23.20.7391-7402.2003. Mol Cell Biol. 2003. PMID: 14517306 Free PMC article. - Cadherin-catenin complex: protein interactions and their implications for cadherin function.
Aberle H, Schwartz H, Kemler R. Aberle H, et al. J Cell Biochem. 1996 Jun 15;61(4):514-23. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. J Cell Biochem. 1996. PMID: 8806074 Review. - Analysis of desmosomal cadherin-adhesive function and stoichiometry of desmosomal cadherin-plakoglobin complexes.
Kowalczyk AP, Borgwardt JE, Green KJ. Kowalczyk AP, et al. J Invest Dermatol. 1996 Sep;107(3):293-300. doi: 10.1111/1523-1747.ep12363000. J Invest Dermatol. 1996. PMID: 8751959 Review.
Cited by
- Tumor suppressor PTEN inhibits nuclear accumulation of beta-catenin and T cell/lymphoid enhancer factor 1-mediated transcriptional activation.
Persad S, Troussard AA, McPhee TR, Mulholland DJ, Dedhar S. Persad S, et al. J Cell Biol. 2001 Jun 11;153(6):1161-74. doi: 10.1083/jcb.153.6.1161. J Cell Biol. 2001. PMID: 11402061 Free PMC article. - Trenbolone enhances myogenic differentiation by enhancing β-catenin signaling in muscle-derived stem cells of cattle.
Zhao JX, Hu J, Zhu MJ, Du M. Zhao JX, et al. Domest Anim Endocrinol. 2011 May;40(4):222-9. doi: 10.1016/j.domaniend.2011.01.004. Epub 2011 Feb 26. Domest Anim Endocrinol. 2011. PMID: 21402455 Free PMC article. - Changing roles of cadherins and catenins during progression of squamous intraepithelial lesions in the uterine cervix.
de Boer CJ, van Dorst E, van Krieken H, Jansen-van Rhijn CM, Warnaar SO, Fleuren GJ, Litvinov SV. de Boer CJ, et al. Am J Pathol. 1999 Aug;155(2):505-15. doi: 10.1016/S0002-9440(10)65146-2. Am J Pathol. 1999. PMID: 10433943 Free PMC article. - The identification and characterization of the p.G91 deletion in CRYBA1 in a Chinese family with congenital cataracts.
Li D, Jing Q, Jiang Y. Li D, et al. BMC Med Genet. 2019 Sep 5;20(1):153. doi: 10.1186/s12881-019-0882-z. BMC Med Genet. 2019. PMID: 31488069 Free PMC article. - Amyloid-beta binds to the extracellular cysteine-rich domain of Frizzled and inhibits Wnt/beta-catenin signaling.
Magdesian MH, Carvalho MM, Mendes FA, Saraiva LM, Juliano MA, Juliano L, Garcia-Abreu J, Ferreira ST. Magdesian MH, et al. J Biol Chem. 2008 Apr 4;283(14):9359-68. doi: 10.1074/jbc.M707108200. Epub 2008 Jan 30. J Biol Chem. 2008. PMID: 18234671 Free PMC article.
References
- Aberle H, Bierkamp C, Torchard D, Serova O, Wagener T, Natt E, Wirsching J, Heidkämper C, Montagna M, Lynch HT, et al. The human plakoglobin gene localizes on chromosome 17q21 and is subject to loss of heterozygosity in breast and ovarian cancer. Proc Natl Acad Sci USA. 1995;92:6384–6388. - PMC - PubMed
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
- Ben-Ze'ev A. Cytoskeletal and adhesion proteins as tumor suppressors. Curr Opin Cell Biol. 1997;9:99–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials