Human telomerase contains evolutionarily conserved catalytic and structural subunits - PubMed (original) (raw)
Human telomerase contains evolutionarily conserved catalytic and structural subunits
L Harrington et al. Genes Dev. 1997.
Abstract
We have cloned and characterized a human gene encoding TP2 (telomerase-associated protein 2), a protein with similarity to reverse transcriptases and the catalytic telomerase subunits from Saccharomyces cerevisiae and Euplotes aediculatus. Indirect immunofluorescence revealed that TP2 was localized to the nucleus. Using antibodies to endogenous and epitope-tagged TP2, we found that TP2 was associated specifically with human telomerase activity and the recently identified telomerase-associated protein TP1. Mutation of conserved residues within the reverse transcriptase domain of TP2 severely reduced associated telomerase activity. These results suggest that telomerase is an evolutionarily conserved multisubunit complex composed of both structural and catalytic subunits.
Figures
Figure 1
Amino acid sequence of human TP2. (A) Predicted amino acid sequence of human TP2. Homology to the RT domain is boxed with a solid line. (B) Domain of highest homology between TP2, p123, and Est2. Alignment was generated by Clustal W using a PAM350 matrix, then adjusted manually. Residues conserved in at least two of the aligned proteins are shown in boldface type. Residues conserved among RT are boxed, with consensus residues shown below.
Figure 2
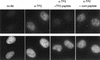
Immunofluorescence of TP2 protein. HeLa cells were stained with DAPI and anti-TP2 or control sera as indicated: (no Ab) No primary antibody was added; (α-TP2) the TP2 antipeptide antibody; (α-TP2 + TP2 peptide) antibody incubated with TP2 peptide; (α-TP2 + cont peptide) antibody incubated with nonspecific peptide (see Materials and Methods).
Figure 3
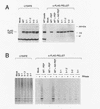
TP2 associates with telomerase activity in HeLa cells. (A) HeLa cell lysate (5 μg) (lysate) was assayed for telomerase activity by the TRAP protocol in the absence (HeLa) and presence (+RNase) of ribonuclease. Telomerase activity of supernatants after incubation (IP supernatant) with the control antibodies (α-Myc and α-GST), anti-TP2 peptide antiserum (α-TP2), and anti-TP2 peptide antiserum incubated with 30 and 60 μg each of nonspecific (non-spec.) or TP2-specific peptide (specific). Under these conditions, the TP2 antisera does not significantly immunodeplete telomerase acitivity from the supernatant. (B) The immunoprecipitates as shown in A were assayed for telomerase activity using TRAP in the absence (−) and presence (+) of RNase A. (C) The remainder of the immunoprecipitates shown in B were analyzed by Westen blotting with the anti-TP2 peptide antiserum. The position of the protein markers are indicated at right in kD.
Figure 4
Analysis of mutations in the putative catalytic residues of TP2. (A) 293 cells were transiently transfected with no plasmid (Mock), wild-type TP2 (WT), and the following TP2 point mutations in the third and fifth conserved RT motifs: 5-1, 5-1.2, 5-2, 3-1 (see Materials and Methods). Cell lysates were immunoprecipitated with anti-FLAG antibody, and the lysates (LYSATE) and immunoprecipitates (α-FLAG PELLET) were resolved by SDS-PAGE and probed with TP2 antiserum. The specificity of the FLAG immunoprecipitate for tagged TP2 was demonstrated by competiton of FLAG-tagged, wild-type TP2 as follows: WT − PEP, no peptide added; WT + PEP, preincubation with FLAG peptide; and WT + NS PEP, preincubation with a nonspecific (TP1) peptide. (B) The samples as in A were analyzed for telomerase activity. The anti-FLAG immunoprecipitates were assayed in the absence (−) and presence (+) of RNase A.
Figure 5
TP2 interacts with TP1 in HeLa cells and transfected neuroblastoma cells. (A) Neuroblastoma cells were mock-transfected (N2A Mock) or transfected with MYC–TP1 alone, FLAG–TP2 alone, or MYC–TP1 and FLAG–TP2. Lysates plus (+) or minus (−) RNase were immunoprecipitated with anti-FLAG antibody, the precipitates were resolved by SDS-PAGE, and the Western blot was probed with anti-Myc and anti-FLAG antiserum. The peptide competitions on the FLAG immunoprecipitates were −PEP, no peptide added; +PEP, plus FLAG peptide; and +NS PEP, plus nonspecific peptide (Myc peptide). The mobility of the protein markers is shown at right. (B) Immunoprecipitations with TP2 (α-TP2) or control antiserum (rabbit α-mouse; control) and anti-GST (α-GST) were assayed for telomerase activity (top panel), Western blotting using anti-TP2 antiserum (middle), and anti-TP1 antiserum (bottom). Immunoprecipitation with anti-TP2 antiserum was also performed in the presence of TP2-specific peptide (α-TP2 + pep) and two different nonspecific peptides (to TP1; see Materials and Methods). In the bottom two panels, the samples are in the same order as above; the position of the protein markers are indicated at right, and the endogenous TP1 and TP2 proteins are indicated at left of each panel.
References
- Blasco MA, Funk W, Villeponteau B, Greider CW. Functional characterization and developmental regulation of mouse telomerase RNA. Science. 1995;269:1267–1270. - PubMed
- Bryan TM, Reddel RR. Telomere dynamics and telomerase activity in in vitro immortalized cell lines. Eur J Canc. 1997;33:767–773. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases