Regulation of amyloid precursor protein catabolism involves the mitogen-activated protein kinase signal transduction pathway - PubMed (original) (raw)
Regulation of amyloid precursor protein catabolism involves the mitogen-activated protein kinase signal transduction pathway
J Mills et al. J Neurosci. 1997.
Abstract
Catabolic processing of the amyloid precursor protein (APP) is subject to regulatory control by protein kinases. We hypothesized that this regulation involves sequential activation of the enzymes mitogen-activated protein kinase kinase (MEK) and extracellular signal-regulated protein kinase (ERK). In the present investigation, we provide evidence that MEK is critically involved in regulating APP processing by both nerve growth factor and phorbol esters. Western blot analysis of the soluble N-terminal APP derivative APPs demonstrated that the synthetic MEK inhibitor PD 98059 antagonized nerve growth factor stimulation of both APPs production and ERK activation in PC12 cells. Moreover, PD 98059 inhibited phorbol ester stimulation of APPs production and activation of ERK in both human embryonic kidney cells and cortical neurons. Furthermore, overexpression of a kinase-inactive MEK mutant inhibited phorbol ester stimulation of APP secretion and activation of ERK in human embryonic kidney cell lines. Most important, PD 98059 antagonized phorbol ester-mediated inhibition of Abeta secretion from cells overexpressing human APP695 carrying the "Swedish mutation." Taken together, these data indicate that MEK and ERK may be critically involved in protein kinase C and nerve growth factor regulation of APP processing. The mitogen-activated protein kinase cascade may provide a novel target for altering catabolic processing of APP.
Figures
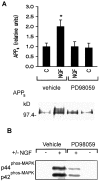
Fig. 1.
PD 98059 inhibits NGF receptor stimulation of APPs secretion and ERK activation in PC12 cells.A, Top, Densitometric analysis of the effect of NGF (100 ng/ml) on basal APPs release with or without PD 98059 (10 μ
m
). Data are mean ± SEM of three experiments (*p < 0.05, different from all other treatment groups). Bottom, Representative Western blot of APPs fragments released in 15 min by PC12 cells alone or in the presence of NGF with or without PD 98059.B, Representative Western blot of phospho-ERK in PC12 cells after a 15 min drug exposure. The increase in immunoreactivity of the phospho-ERK-specific antibody in the presence of NGF was inhibited by PD 98059.
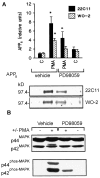
Fig. 2.
PD 98059 antagonizes phorbol ester stimulation of APPs release in 15 min and ERK activation in HEK 293 cells.A, Top, Densitometric analysis of PMA (0.1 μ
m
) stimulation of APPs secretion with or without PD 98059 (10 μ
m
). Data are mean ± SEM and represent five experiments for 22C11 (filled columns) or three experiments for WO-2 (hatched columns) (*p < 0.05, different from all other treatment groups). Bottom, Representative Western blot of the effect of PMA on basal APPs release alone or in the presence of PD 98059. B, Representative Western blot of ERK isoforms with ERK1 C terminus antibody (top) or phospho-ERK forms (bottom) in HEK 293 cells after a 15 min drug exposure. The PMA-induced “electrophoretic shift” was inhibited by PD 98059. Similarly, the increase in phospho-ERK immunoreactivity in the presence of PMA was antagonized by PD 98059.
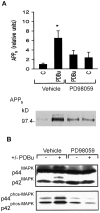
Fig. 3.
PD 98059 inhibits PKC stimulation of APPs secretion and ERK activation in cortical neurons.A, Top, Densitometric analysis of PDBu (1 μ
m
) stimulation of APPs secretion in rat cortical cultures with or without PD 98059 (10 μ
m
). Data are mean ± SEM of five experiments (*p < 0.05, different from all other treatment groups).Bottom, Representative Western blot of the effect of PD 98059 on PDBu stimulation of APPs release in 15 min.B, Representative Western blot of ERK isoforms with ERK C terminus antibody (top) or phospho-ERK forms (bottom) in cortical cultures after a 1 hr drug exposure: phorbol ester-induced increase in the phosphorylation state of ERK was antagonized by pharmacological inhibition of MEK.
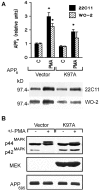
Fig. 4.
The kinase-dead mutant (K97A) inhibits phorbol ester stimulation of APPs secretion and ERK activation in HEK 293 cells. A, Top, Densitometric analysis of PMA (0.1 μ
m
) stimulation of APPs secretion in cells expressing the MEK mutant (K97A) or vector alone (Vector). Data are mean ± SEM and represent three experiments for both 22C11 (filled columns) and WO-2 (hatched columns) (*p < 0.05, different from all other treatment groups). Bottom, Representative Western blot of the effect of PMA on basal APPs release after transient transfection of vector alone or the K97A mutant.B, Top, Representative Western blot of ERK isoforms with an ERK C terminus antibody in HEK 293 cells transfected with the kinase-dead MEK mutant or vector alone. The “electrophoretic shift” induced by PMA treatment in cells expressing vector alone was inhibited in cells expressing the kinase-dead MEK mutant. Middle, Representative Western blot of MEK1 using a rabbit polyclonal antibody raised against the N terminus of MEK1. Bottom, Representative Western blot of cellular APP using a monoclonal antibody generated against the N terminus of APP.
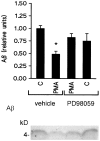
Fig. 5.
PD 98059 antagonizes phorbol ester inhibition of Aβ secretion in K695sw cells. Top, Densitometric analysis of PMA (1 μ
m
) inhibition of Aβ secretion in K695sw cells with or without PD 98059 (10 μ
m
). Data are mean ± SEM of seven experiments [*p < 0.05, different from control (vehicle alone)]. Bottom, Representative Western blot of the effects of the MEK antagonist PD 98059 on PMA inhibition of Aβ release.
Similar articles
- Regulation of secretion of Alzheimer amyloid precursor protein by the mitogen-activated protein kinase cascade.
Desdouits-Magnen J, Desdouits F, Takeda S, Syu LJ, Saltiel AR, Buxbaum JD, Czernik AJ, Nairn AC, Greengard P. Desdouits-Magnen J, et al. J Neurochem. 1998 Feb;70(2):524-30. doi: 10.1046/j.1471-4159.1998.70020524.x. J Neurochem. 1998. PMID: 9453546 - Mitogen-activated protein kinase-dependent and protein kinase C-dependent pathways link the m1 muscarinic receptor to beta-amyloid precursor protein secretion.
Haring R, Fisher A, Marciano D, Pittel Z, Kloog Y, Zuckerman A, Eshhar N, Heldman E. Haring R, et al. J Neurochem. 1998 Nov;71(5):2094-103. doi: 10.1046/j.1471-4159.1998.71052094.x. J Neurochem. 1998. PMID: 9798935 - The regulation of amyloid precursor protein metabolism by cholinergic mechanisms and neurotrophin receptor signaling.
Rossner S, Ueberham U, Schliebs R, Perez-Polo JR, Bigl V. Rossner S, et al. Prog Neurobiol. 1998 Dec;56(5):541-69. doi: 10.1016/s0301-0082(98)00044-6. Prog Neurobiol. 1998. PMID: 9775403 Review. - New players in old amyloid precursor protein-processing pathways.
Rossner S. Rossner S. Int J Dev Neurosci. 2004 Nov;22(7):467-74. doi: 10.1016/j.ijdevneu.2004.07.004. Int J Dev Neurosci. 2004. PMID: 15465276 Review.
Cited by
- Copper inhibits beta-amyloid production and stimulates the non-amyloidogenic pathway of amyloid-precursor-protein secretion.
Borchardt T, Camakaris J, Cappai R, Masters CL, Beyreuther K, Multhaup G. Borchardt T, et al. Biochem J. 1999 Dec 1;344 Pt 2(Pt 2):461-7. Biochem J. 1999. PMID: 10567229 Free PMC article. - The identification and characterization of oxidized RNAs in Alzheimer's disease.
Shan X, Tashiro H, Lin CL. Shan X, et al. J Neurosci. 2003 Jun 15;23(12):4913-21. doi: 10.1523/JNEUROSCI.23-12-04913.2003. J Neurosci. 2003. PMID: 12832513 Free PMC article. - AMPA receptor activation promotes non-amyloidogenic amyloid precursor protein processing and suppresses neuronal amyloid-β production.
Hoey SE, Buonocore F, Cox CJ, Hammond VJ, Perkinton MS, Williams RJ. Hoey SE, et al. PLoS One. 2013 Oct 24;8(10):e78155. doi: 10.1371/journal.pone.0078155. eCollection 2013. PLoS One. 2013. PMID: 24205136 Free PMC article. - APP Binds to the EGFR Ligands HB-EGF and EGF, Acting Synergistically with EGF to Promote ERK Signaling and Neuritogenesis.
da Rocha JF, Bastos L, Domingues SC, Bento AR, Konietzko U, da Cruz E Silva OAB, Vieira SI. da Rocha JF, et al. Mol Neurobiol. 2021 Feb;58(2):668-688. doi: 10.1007/s12035-020-02139-2. Epub 2020 Oct 2. Mol Neurobiol. 2021. PMID: 33009641 - Obesity, leptin, and Alzheimer's disease.
Lee EB. Lee EB. Ann N Y Acad Sci. 2011 Dec;1243:15-29. doi: 10.1111/j.1749-6632.2011.06274.x. Ann N Y Acad Sci. 2011. PMID: 22211890 Free PMC article. Review.
References
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
- Anderson JP, Esch FS, Keim PS, Sambamurti K, Lieberburg I, Robakis NK. Exact cleavage site of Alzheimer amyloid precursor in neuronal PC-12 cells. Neurosci Lett. 1991;128:126–128. - PubMed
- Anderson NG, Maller JL, Tonks NK, Sturgill TW. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990;343:651–653. - PubMed
- Aplin AE, Gibb GM, Jacobsen S, Gallo J-M, Anderton BH. In vitro phosphorylation of the cytoplasmic domain of the amyloid precursor protein by glycogen synthase kinase-3β. J Neurochem. 1996;76:699–707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous