Myosin VIIA is required for aminoglycoside accumulation in cochlear hair cells - PubMed (original) (raw)
Myosin VIIA is required for aminoglycoside accumulation in cochlear hair cells
G P Richardson et al. J Neurosci. 1997.
Abstract
Myosin VIIA is expressed by sensory hair cells and has a primary structure predicting a role in membrane trafficking and turnover, processes that may underlie the susceptibility of hair cells to aminoglycoside antibiotics. [3H]Gentamicin accumulation and the effects of aminoglycosides were therefore examined in cochlear cultures of mice with different missense mutations in the myosin VIIA gene, Myo7a, to see whether myosin VIIA plays a role in aminoglycoside ototoxicity. Hair cells from homozygous mutant Myo7ash1 mice, with a mutation in a nonconserved region of the myosin VIIA head, respond rapidly to aminoglycoside treatment and accumulate high levels of gentamicin. Hair cells from homozygous mutant Myo7a6J mice, with a mutation at a highly conserved residue close to the ATP binding site of the myosin VIIA head, do not accumulate [3H]gentamicin and are protected from aminoglycoside ototoxicity. Hair cells from heterozygotes of both alleles accumulate [3H]gentamicin and respond to aminoglycosides. Although aminoglycoside uptake is thought to be via apical surface-associated endocytosis, coated pit numbers on the apical membrane of heterozygous and homozygous Myo7a6J hair cells are similar. Pulse-chase experiments with cationic ferritin confirm that the apical endocytotic pathway is functional in homozygous Myo7a6J hair cells. Transduction currents can be recorded from both heterozygous and homozygous Myo7a6J hair cells, suggesting it is unlikely that the drug enters via diffusion through the mechanotransducer channel. The results show that myosin VIIA is required for aminoglycoside accumulation in hair cells. Myosin VIIA may transport a putative aminoglycoside receptor to the hair cell surface, indirectly translocate it to sites of membrane retrieval, or retain it in the endocytotic pathway.
Figures
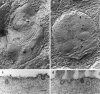
Fig. 1.
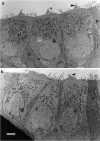
Transmission electron micrographs of basal-coil outer hair cells in the organ of Corti in cultures from heterozygous (a) and homozygous (b)Myo7a 6J mice. The profiles of three outer hair cell bodies can be observed in both _a_and b, which are flanked by the darker staining processes of the immature Deiters’ (D) and pillar (P) cells. The _arrowheads_indicate the stereocilia bundles on the first row outer hair cells. Scale bar, 2 μm.
Fig. 2.
Scanning electron micrographs of the apical surfaces of basal-coil outer hair cells in the organ of Corti in cultures from heterozygous (a) and homozygous (b) Myo7a _6J_mice. In a, three rows of well organized, V-shaped stereocilia bundles are observed surrounded by supporting cell microvilli. In b, the hair cell surfaces are larger and, the stereocilia bundles are disorganized. Scale bar, 5 μm.
Fig. 3.
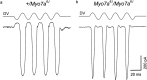
Transducer currents recorded from outer hair cells in cultures prepared from heterozygous (a) and homozygous (b)Myo7a 6J mice. Currents were recorded in response to 45 Hz sinusoidal force stimuli at a holding potential of −84 mV. The driver voltage signal (DV; amplitude, 45 V) to the fluid jet is shown above each current record. Positive deflections are excitatory. Responses are averages of four (a) and five (b) repetitions. Recordings were made at 22°C.
Fig. 4.
Scanning electron micrographs of the apical surfaces of basal-coil outer hair cells in the organ of Corti of cultures from heterozygous (a) and homozygous (b) Myo7a _6J_mice that have been incubated in HBHBSS containing 1 m
m
neomycin sulfate for 1 hr at room temperature. In a, note the large blisters in the vicinity of the kinocilium (double arrowheads) and the smaller blebs (single arrows) around the perimeter of each hair cell. The apical surface of the hair cells in b is larger but shows little damage. Scale bars, 5 μm.
Fig. 5.
Toluidine blue-stained semithin sections of basal-coil cultures from heterozygous (a, c) and homozygous (b, d)Myo7a 6J mice that have been incubated in either HBHBSS (a, b) or HBHBSS containing 2 m
m
gentamicin sulfate (c, d) for 2 hr at 37°C. In b, the large arrowhead points to the single inner hair cell, and the smaller arrowheads point to the 3 outer hair cells. In_a_, G indicates the greater epithelial ridge. In c, the small arrows point to nuclei with condensed chromatin. Scale bar, 50 μm.
Fig. 6.
Autoradiographs of sections from basal-coil cochlear cultures prepared from heterozygous (a) and homozygous (b)Myo7a 6J mice that have been labeled with 0.1 m
m
[3H]gentamicin for 2 hr at 37°C. C, Connective tissue; G, greater epithelial ridge; T, tectorial membrane;S, collagen substrate (in a). In_b_, the large arrowhead points to the basal pole of the inner hair cell, and the small arrowheads point to those of the three outer hair cells. Scale bar, 50 μm.
Fig. 7.
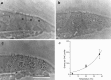
Autoradiographs of basal-coil cochlear hair cells in heterozygous Myo7a 6J cultures labeled with 0.1 m
m
[3H]gentamicin for 2 hr at 3°C (a), 20°C (b), and 37°C (c).I, Inner hair cell; O, outer hair cell (in a). Scale bar, 50 μm. The average numbers of grains (±SD; n = 18 at 3 and 20°C;n = 17 at 37°C) per hair cell profile at each temperature are shown in d. The fitted curve is of the form N =A_exp(B/(t + 273)), where_N is the number of grains, t is the temperature in degrees Celsius, and A (1.30 × 1010) and B (−5602°C) are constants.
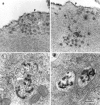
Fig. 8.
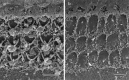
(a, b) Freeze fracture replicas of the apical surface of outer hair cells in cultures prepared from heterozygous (a) and homozygous (b) Myo7a 6J_mice. The arrowheads in a and_b point to invaginations of the apical surface corresponding to sites of coated vesicle formation. Scale bar, 1 μm.c, d, Transmission electron micrographs illustrating examples of coated pits present on the apical surfaces of outer hair cells in cultures prepared from heterozygous (c) and homozygous (d)Myo7a 6J mice. Scale bar, 0.1 μm.
Fig. 9.
Transmission electron micrographs illustrating examples of cationic ferritin-labeled tubular and vesicular profiles (a, b) and multivesicular bodies (c, d) in outer hair cells from cultures prepared from heterozygous (a, c) and homozygous (b, d)Myo7a 6J mice. The_arrowheads_ in a indicate the apical membrane of the outer hair cell. Scale bar, 0.1 μm.
Fig. 10.
a, b Scanning electron micrographs showing the apical surface of cultures from heterozygous (a) and homozygous (b)Myo7a sh1 mice after treatment with 1 m
m
neomycin for 1 hr at room temperature. c, d, Toluidine blue-stained semithin sections of cultures prepared from heterozygous (c) and homozygous (d)Myo7a sh1 mice that have been treated with 2 m
m
gentamicin sulfate for 2 hr at 37°C. The small arrows point to nuclei with condensed chromatin. e, f, Autoradiographs of cultures from heterozygous (e) and homozygous (f)Myo7a sh1 mice that have been labeled with 0.1 m
m
[3H]gentamicin for 2 hr at 37°C. Scale bars: a, b, 10 μm:c–f, 50 μm.
Similar articles
- A missense mutation in myosin VIIA prevents aminoglycoside accumulation in early postnatal cochlear hair cells.
Richardson GP, Forge A, Kros CJ, Marcotti W, Becker D, Williams DS, Thorpe J, Fleming J, Brown SD, Steel KP. Richardson GP, et al. Ann N Y Acad Sci. 1999 Nov 28;884:110-24. Ann N Y Acad Sci. 1999. PMID: 10842588 - Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells.
Self T, Mahony M, Fleming J, Walsh J, Brown SD, Steel KP. Self T, et al. Development. 1998 Feb;125(4):557-66. doi: 10.1242/dev.125.4.557. Development. 1998. PMID: 9435277 - Unconventional myosins and the genetics of hearing loss.
Friedman TB, Sellers JR, Avraham KB. Friedman TB, et al. Am J Med Genet. 1999 Sep 24;89(3):147-57. doi: 10.1002/(sici)1096-8628(19990924)89:3<147::aid-ajmg5>3.0.co;2-6. Am J Med Genet. 1999. PMID: 10704189 Review. - Entry of aminoglycosides into renal tubular epithelial cells via endocytosis-dependent and endocytosis-independent pathways.
Nagai J, Takano M. Nagai J, et al. Biochem Pharmacol. 2014 Aug 15;90(4):331-7. doi: 10.1016/j.bcp.2014.05.018. Epub 2014 May 29. Biochem Pharmacol. 2014. PMID: 24881578 Review.
Cited by
- EGFR signaling is required for regenerative proliferation in the cochlea: conservation in birds and mammals.
White PM, Stone JS, Groves AK, Segil N. White PM, et al. Dev Biol. 2012 Mar 1;363(1):191-200. doi: 10.1016/j.ydbio.2011.12.035. Epub 2012 Jan 2. Dev Biol. 2012. PMID: 22230616 Free PMC article. - Screen of FDA-approved drug library reveals compounds that protect hair cells from aminoglycosides and cisplatin.
Vlasits AL, Simon JA, Raible DW, Rubel EW, Owens KN. Vlasits AL, et al. Hear Res. 2012 Dec;294(1-2):153-65. doi: 10.1016/j.heares.2012.08.002. Epub 2012 Aug 31. Hear Res. 2012. PMID: 22967486 Free PMC article. - Generation of inner ear hair cells by direct lineage conversion of primary somatic cells.
Menendez L, Trecek T, Gopalakrishnan S, Tao L, Markowitz AL, Yu HV, Wang X, Llamas J, Huang C, Lee J, Kalluri R, Ichida J, Segil N. Menendez L, et al. Elife. 2020 Jun 30;9:e55249. doi: 10.7554/eLife.55249. Elife. 2020. PMID: 32602462 Free PMC article. - Identifying targets to prevent aminoglycoside ototoxicity.
Kim J, Hemachandran S, Cheng AG, Ricci AJ. Kim J, et al. Mol Cell Neurosci. 2022 May;120:103722. doi: 10.1016/j.mcn.2022.103722. Epub 2022 Mar 24. Mol Cell Neurosci. 2022. PMID: 35341941 Free PMC article. Review. - Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity.
Alharazneh A, Luk L, Huth M, Monfared A, Steyger PS, Cheng AG, Ricci AJ. Alharazneh A, et al. PLoS One. 2011;6(7):e22347. doi: 10.1371/journal.pone.0022347. Epub 2011 Jul 26. PLoS One. 2011. PMID: 21818312 Free PMC article.
References
- Avraham KB, Hasson T, Steel KP, Kingsley DM, Russell LB, Mooseker MS, Copeland NG, Jenkins NA. The mouse Snell’s waltzer deafness gene encodes an unconventional myosin required for the structural integrity of inner ear hair cells. Nat Genet. 1995;11:369–375. - PubMed
- Cojecel C, Hook JB. Aminoglycoside nephrotoxicity. Trends Pharmacol Sci. 1983;4:174–179.
- Chen Z-Y, Hasson T, Kelley PM, Schwender BJ, Schwartz MF, Ramakrishnan M, Kimberling WJ, Mooseker MS, Corey DP. Molecular cloning and domain structure of human myosin-VIIA, the gene product defective in Usher syndrome 1B. Genomics. 1996;36:440–448. - PubMed
- Darrouzet J, Guilhaume A. Ototoxicité de la kanamycine au jour le jour. Etude experimentale en microscopie électronique. Rev Laryngol Otol Rhinol (Bord) 1974;95:601–621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical