Sublethal oxygen-glucose deprivation alters hippocampal neuronal AMPA receptor expression and vulnerability to kainate-induced death - PubMed (original) (raw)
Sublethal oxygen-glucose deprivation alters hippocampal neuronal AMPA receptor expression and vulnerability to kainate-induced death
H S Ying et al. J Neurosci. 1997.
Abstract
Recent studies have suggested that rats subjected to transient global brain ischemia develop depressed expression of GluR-B in CA1 hippocampal neurons. The present study was performed to determine whether a similar change in AMPA receptor expression could be triggered in vitro by sublethal oxygen-glucose deprivation in rat hippocampal neuronal cultures. mRNA was extracted from individual hippocampal neurons via patch electrodes and amplified by RT-PCR 24-48 hr after sublethal oxygen-glucose deprivation. Compared with controls, insulted neurons expressed increased levels of GluR-D flop. As an indication that this change in receptor expression was functionally significant, insulted cultures exhibited increased AMPA- or kainate-induced 45Ca2+ accumulation sensitive to Joro spider toxin and increased vulnerability to kainate-induced death. These data support the hypothesis that exposure to ischemia may enhance subsequent hippocampal neuronal vulnerability to AMPA receptor-mediated excitotoxicity by modifying the relative expression of AMPA receptor subunits in a manner that promotes Ca2+ permeability.
Figures
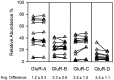
Fig. 1.
Amplification and detection of AMPA receptors by single cell RT-PCR is reproducible. Cytoplasmic contents from 13 cells were aspirated via patch electrode and reverse-transcribed as described in Materials and Methods. Then, half (5 μl) of each product was aliquoted into a fresh PCR tube and amplified and detected separately. ▴ indicate relative mRNA abundance for each subunit;left, as determined in the original tubes;right, as determined in the second aliquots. A_line_ connects samples drawn from the same cell. Average difference refers to the mean shift in relative abundance between the original and aliquoted samples for that subunit.
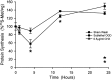
Fig. 2.
Protein synthesis inhibition after sublethal oxygen–glucose deprivation (OGD) is mild and transient. Sister cultures were labeled with [35S]methionine for 1 hr epochs either before (0 hr) or at the indicated times after (•) sham wash, (▪) 30 min sublethal OGD, or (▴) continuous application of 0.5 μg/ml cycloheximide (mean ± SEM; n = 8 cultures). Asterisks indicate difference from sham wash at corresponding time point; p < 0.05 using two-tailed Student’s t test.
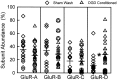
Fig. 3.
Sublethal oxygen–glucose deprivation (OGD) increases GluR-D mRNA relative abundance in cultured hippocampal neurons. Relative mRNA abundance for each subunit in a single cell for sham-washed controls (♦; n = 18 cells) or for OGD conditioned cells (▴; n = 23 cells) show large variance in each group. Cross-hairs are placed at the mean for each experimental group, and circles were drawn around four cells that appeared atypical. Asterisk_indicates difference from corresponding sham wash control at_p = 0.006, using two-tailed Student’s_t_ test.
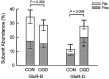
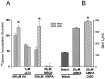
Fig. 4.
Flip/flop analysis comparing sham-washed controls with oxygen–glucose deprivation (OGD) conditioned cells demonstrated that the increase in GluR-D was caused by an increase in GluR-D flop (bars show mean ± SEM abundance for each flip or flop subtype; n = 18–23).Asterisk indicates difference from corresponding control flop subunit at p < 0.05, using two-tailed Student’s t test.
Fig. 5.
Cultures exposed to sublethal oxygen–glucose deprivation (OGD) exhibit increased AMPA/kainate-activated 45Ca2+accumulation and AMPA-activated Δ [Ca2+]i in neurons. A,45Ca2+ accumulation during a 5 min exposure to 500 μ
m
kainate or 300 μ
m
AMPA in either sham-washed controls or cultures exposed to sublethal OGD 24 hr earlier (mean ± SEM; n = 8–14 cultures). Both kainate- and AMPA-activated 45Ca2+accumulation were potentiated by sublethal OGD, and the kainate-induced potentiation was eliminated by 1 μ
m
Joro spider toxin.B, Sublethal OGD also potentiated the increase in [Ca2+]i evoked by a 15 sec exposure to 20 μ
m
AMPA (mean ± SEM; n = 122–137 cells from 4 separate experiments). Basal [Ca2+]i levels in neurons exposed to sublethal OGD were not different from those exposed to sham wash (data not shown). Asterisks indicate difference from respective sham-washed control at p < 0.05, using two-tailed Student’s t test.
Similar articles
- AMPA receptor-mediated excitotoxicity in human NT2-N neurons results from loss of intracellular Ca2+ homeostasis following marked elevation of intracellular Na+.
Itoh T, Itoh A, Horiuchi K, Pleasure D. Itoh T, et al. J Neurochem. 1998 Jul;71(1):112-24. doi: 10.1046/j.1471-4159.1998.71010112.x. J Neurochem. 1998. PMID: 9648857 - Neuropeptide Y release from cultured hippocampal neurons: stimulation by glutamate acting at N-methyl-D-aspartate and AMPA receptors.
Gemignani A, Marchese S, Fontana G, Raiteri M. Gemignani A, et al. Neuroscience. 1997 Nov;81(1):23-31. doi: 10.1016/s0306-4522(97)00168-1. Neuroscience. 1997. PMID: 9300398 - Voltage-dependent blockage of Ca(2+)-permeable AMPA receptors by joro spider toxin in cultured rat hippocampal neurones.
Iino M, Koike M, Isa T, Ozawa S. Iino M, et al. J Physiol. 1996 Oct 15;496 ( Pt 2)(Pt 2):431-7. doi: 10.1113/jphysiol.1996.sp021696. J Physiol. 1996. PMID: 8910227 Free PMC article. - AMPA receptor calcium permeability, GluR2 expression, and selective motoneuron vulnerability.
Vandenberghe W, Robberecht W, Brorson JR. Vandenberghe W, et al. J Neurosci. 2000 Jan 1;20(1):123-32. doi: 10.1523/JNEUROSCI.20-01-00123.2000. J Neurosci. 2000. PMID: 10627588 Free PMC article. - Pharmacological and molecular characterization of glutamate receptors in the MIN6 pancreatic beta-cell line.
Morley P, MacLean S, Gendron TF, Small DL, Tremblay R, Durkin JP, Mealing G. Morley P, et al. Neurol Res. 2000 Jun;22(4):379-85. doi: 10.1080/01616412.2000.11740687. Neurol Res. 2000. PMID: 10874687
Cited by
- Ischemic insults direct glutamate receptor subunit 2-lacking AMPA receptors to synaptic sites.
Liu B, Liao M, Mielke JG, Ning K, Chen Y, Li L, El-Hayek YH, Gomez E, Zukin RS, Fehlings MG, Wan Q. Liu B, et al. J Neurosci. 2006 May 17;26(20):5309-19. doi: 10.1523/JNEUROSCI.0567-06.2006. J Neurosci. 2006. PMID: 16707783 Free PMC article. - The influence of glutamate receptor 2 expression on excitotoxicity in Glur2 null mutant mice.
Iihara K, Joo DT, Henderson J, Sattler R, Taverna FA, Lourensen S, Orser BA, Roder JC, Tymianski M. Iihara K, et al. J Neurosci. 2001 Apr 1;21(7):2224-39. doi: 10.1523/JNEUROSCI.21-07-02224.2001. J Neurosci. 2001. PMID: 11264298 Free PMC article. - Protein-protein coupling/uncoupling enables dopamine D2 receptor regulation of AMPA receptor-mediated excitotoxicity.
Zou S, Li L, Pei L, Vukusic B, Van Tol HH, Lee FJ, Wan Q, Liu F. Zou S, et al. J Neurosci. 2005 Apr 27;25(17):4385-95. doi: 10.1523/JNEUROSCI.5099-04.2005. J Neurosci. 2005. PMID: 15858065 Free PMC article. - Translational downregulation of the noncatalytic growth factor receptor TrkB.T1 by ischemic preconditioning of primary neurons.
Steinbeck JA, Methner A. Steinbeck JA, et al. Gene Expr. 2005;12(2):99-106. doi: 10.3727/000000005783992142. Gene Expr. 2005. PMID: 15892451 Free PMC article. - Correlating AMPA receptor activation and cleft closure across subunits: crystal structures of the GluR4 ligand-binding domain in complex with full and partial agonists.
Gill A, Birdsey-Benson A, Jones BL, Henderson LP, Madden DR. Gill A, et al. Biochemistry. 2008 Dec 30;47(52):13831-41. doi: 10.1021/bi8013196. Biochemistry. 2008. PMID: 19102704 Free PMC article.
References
- Aizenman E, Lipton SA, Loring RH. Selective modulation of NMDA responses by reduction and oxidation. Neuron. 1989;2:1257–1263. - PubMed
- Albers GW, Goldberg MP, Choi DW. NMDA antagonists: ready for clinical trial in brain ischemia? Ann Neurol. 1989;25:398–403. - PubMed
- Akbarian S, Smith MA, Jones EG. Editing for an AMPA receptor subunit RNA in prefrontal cortex and striatum in Alzheimer’s disease, Huntington’s disease, and schizophrenia. Brain Res. 1995;699:297–304. - PubMed
- Biagi BA, Enyeart JJ. Gadolinium blocks low- and high-threshold calcium currents in pituitary cells. Am J Physiol. 1990;259:C515–520. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous