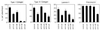Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins - PubMed (original) (raw)
Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins
D R Senger et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Vascular endothelial growth factor (VEGF), also known as vascular permeability factor, is a cytokine of central importance for the angiogenesis associated with cancers and other pathologies. Because angiogenesis often involves endothelial cell (EC) migration and proliferation within a collagen-rich extracellular matrix, we investigated the possibility that VEGF promotes neovascularization through regulation of collagen receptor expression. VEGF induced a 5- to 7-fold increase in dermal microvascular EC surface protein expression of two collagen receptors-the alpha1beta1 and alpha2beta1 integrins-through induction of mRNAs encoding the alpha1 and alpha2 subunits. In contrast, VEGF did not induce increased expression of the alpha3beta1 integrin, which also has been implicated in collagen binding. Integrin alpha1-blocking and alpha2-blocking antibodies (Ab) each partially inhibited attachment of microvascular EC to collagen I, and alpha1-blocking Ab also inhibited attachment to collagen IV and laminin-1. Induction of alpha1beta1 and alpha2beta1 expression by VEGF promoted cell spreading on collagen I gels which was abolished by a combination of alpha1-blocking and alpha2-blocking Abs. In vivo, a combination of alpha1-blocking and alpha2-blocking Abs markedly inhibited VEGF-driven angiogenesis; average cross-sectional area of individual new blood vessels was reduced 90% and average total new vascular area was reduced 82% without detectable effects on the pre-existing vasculature. These data indicate that induction of alpha1beta1 and alpha2beta1 expression by EC is an important mechanism by which VEGF promotes angiogenesis and that alpha1beta1 and alpha2beta1 antagonists may prove effective in inhibiting VEGF-driven angiogenesis in cancers and other important pathologies.
Figures
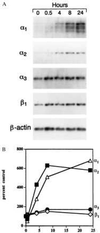
Figure 1
(A) Northern blot analyses of integrin subunit mRNAs in human dermal microvascular EC stimulated with VEGF (20 ng/ml) for up to 24 h. Ten micrograms of total cellular RNA was loaded in each well. (B) Densitometric quantitation of Northern blot analyses. The signal associated with each integrin mRNA was normalized to the internal β-actin mRNA standard to adjust for minor differences in RNA loading.
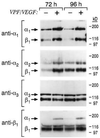
Figure 2
Integrin expression at the surface of dermal microvascular EC following stimulation with VEGF (20 ng/ml) for 72 h and 96 h. Lysates from biotinylated cells were subjected to immunoprecipitation, and immunoprecipitates were subjected to electrophoresis in 7.5% polyacrylamide gels under nonreducing conditions. Control cells were cultured and biotinylated in parallel. As determined by densitometry, α1β1 and α2β1 typically were induced 5- to 7-fold by VEGF treatment.
Figure 3
Attachment assays performed with dermal microvascular EC and integrin-blocking Abs. Cells were stimulated with VEGF (20 ng/ml, 72 h) before assay for maximal induction of α1β1 and α2β1. Control IgG and Abs were used at a concentration of 10 μg/ml.
Figure 4
Spreading of dermal microvascular EC on type I collagen gels after 4 h. Control = unstimulated cells cultured in parallel with cells prestimulated with VEGF (20 ng/ml) for 72 h. A combination of α1-blocking Ab and α2-blocking Ab (10 μg/ml of each) abolished cell spreading of the VEGF prestimulated cells; control IgG (20 μg/ml) was without effect.
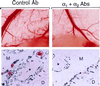
Figure 5
Inhibition of VEGF-driven angiogenesis in adult skin by a combination of α1-blocking Ab and α2-blocking Ab. (Upper) Gross observation after dissection: Matrigel implants, visible at bottom of photographs, together with overlying skin. Small tortuous blood vessels, typical of neovascularization, were visible in the skin adjacent to the implant in animals treated with control Ab (left). These vessels were absent or substantially less visible in animals treated with α1 Ab + α2 Ab (right). In contrast, the larger pre-existing blood vessels appear unaffected by α1 Ab + α2 Ab. (Lower) Light microscopy of paraffin sections: Immunohistochemical staining for CD31 (blue color) illustrates that new blood vessels at the interface between the Matrigel implant (M) and host dermis (D), and in association with large nerves (∗), were reduced in cross-sectional area 90% in the α1 Ab + α2 Ab treated animals (Right), in comparison with controls (Left). Similarly, total new vascular area was reduced 82% (see text). In each panel, two representative vessels are marked with arrows.
Similar articles
- Functional overlap and cooperativity among alphav and beta1 integrin subfamilies during skin angiogenesis.
Perruzzi CA, de Fougerolles AR, Koteliansky VE, Whelan MC, Westlin WF, Senger DR. Perruzzi CA, et al. J Invest Dermatol. 2003 Jun;120(6):1100-9. doi: 10.1046/j.1523-1747.2003.12236.x. J Invest Dermatol. 2003. PMID: 12787141 - The alpha(1)beta(1) and alpha(2)beta(1) integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis.
Senger DR, Perruzzi CA, Streit M, Koteliansky VE, de Fougerolles AR, Detmar M. Senger DR, et al. Am J Pathol. 2002 Jan;160(1):195-204. doi: 10.1016/s0002-9440(10)64363-5. Am J Pathol. 2002. PMID: 11786413 Free PMC article. - Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine.
Brown LF, Detmar M, Claffey K, Nagy JA, Feng D, Dvorak AM, Dvorak HF. Brown LF, et al. EXS. 1997;79:233-69. doi: 10.1007/978-3-0348-9006-9_10. EXS. 1997. PMID: 9002222 Review. - [Vascular endothelial growth factor--fundamental research and experimental study in plastic surgery].
Huang CY, Shen ZY. Huang CY, et al. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2002 Jan;16(1):64-9. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2002. PMID: 11826659 Review. Chinese.
Cited by
- The Added Value of a Collagenated Thermosensitive Bone Substitute as a Scaffold for Bone Regeneration.
Jeanneau C, Catherine JH, Giraud T, Lan R, About I. Jeanneau C, et al. Materials (Basel). 2024 Jan 27;17(3):625. doi: 10.3390/ma17030625. Materials (Basel). 2024. PMID: 38591482 Free PMC article. - Integrins regulation of wound healing processes: insights for chronic skin wound therapeutics.
Yu D, Lu Z, Nie F, Chong Y. Yu D, et al. Front Cell Infect Microbiol. 2024 Mar 5;14:1324441. doi: 10.3389/fcimb.2024.1324441. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38505290 Free PMC article. Review. - Primary Cardiac Angiosarcoma: A Review.
Kumari N, Bhandari S, Ishfaq A, Butt SRR, Ekhator C, Karski A, Kadel B, Altayb Ismail MA, Sherpa TN, Al Khalifa A, Khalifah B, Nguyen N, Lazarevic S, Zaman MU, Ullah A, Yadav V. Kumari N, et al. Cureus. 2023 Jul 16;15(7):e41947. doi: 10.7759/cureus.41947. eCollection 2023 Jul. Cureus. 2023. PMID: 37461430 Free PMC article. Review. - Matrix stiffness, endothelial dysfunction and atherosclerosis.
Xu Z, Chen Y, Wang Y, Han W, Xu W, Liao X, Zhang T, Wang G. Xu Z, et al. Mol Biol Rep. 2023 Aug;50(8):7027-7041. doi: 10.1007/s11033-023-08502-5. Epub 2023 Jun 29. Mol Biol Rep. 2023. PMID: 37382775 Review. - Integrin Targeting Enhances the Antimelanoma Effect of Annexin V in Mice.
Zhu J, Li X, Gao W, Jing J. Zhu J, et al. Int J Mol Sci. 2023 Feb 15;24(4):3859. doi: 10.3390/ijms24043859. Int J Mol Sci. 2023. PMID: 36835282 Free PMC article.
References
- Folkman J, Shing Y. J Biol Chem. 1992;267:10931–10934. - PubMed
- Wilting J, Christ B, Weich H A. Anat Embryol. 1992;186:251–257. - PubMed
- Claffey K P, Brown L F, del Aguila L F, Tognazzi K, Yeo K-T, Manseau E J, Dvorak H F. Cancer Res. 1996;56:172–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA64436/CA/NCI NIH HHS/United States
- R01 CA069184/CA/NCI NIH HHS/United States
- HL54465/HL/NHLBI NIH HHS/United States
- R01 CA064436/CA/NCI NIH HHS/United States
- CA69184/CA/NCI NIH HHS/United States
- R29 CA064436/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases