Platelet-derived growth factor activates mitogen-activated protein kinase in isolated caveolae - PubMed (original) (raw)
Platelet-derived growth factor activates mitogen-activated protein kinase in isolated caveolae
P Liu et al. Proc Natl Acad Sci U S A. 1997.
Abstract
The ability of a peptide hormone to affect many different intracellular targets is thought to be possible because of the modular organization of signal transducing molecules in the cell. Evidence for the presence of signaling modules in metazoan cells, however, is incomplete. Herein we show, with morphology and cell fractionation, that all the components of a mitogen-activated protein kinase pathway are concentrated in caveolae of unstimulated human fibroblasts. Addition of platelet-derived growth factor to either the intact cell or caveolae isolated from these cells stimulates tyrosine phosphorylation and activates mitogen-activated protein kinases in caveolae. The molecular machinery for kinase activation, therefore, is preorganized at the cell surface of quiescent cells.
Figures
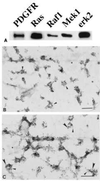
Figure 1
Components of MAP kinase module in caveolae. Normal human fibroblasts were grown as described. (A) Caveolae were purified and immunoblotted with the indicated antibody. (B and C) Membranes were attached to Formvar/carbon-coated electron microscope grids and labeled with either monoclonal anti-erk2 IgG (10-nm gold) alone (B) or monoclonal anti-erk2 IgG (10-nm gold) plus polyclonal anti-PDGF receptor IgG (5-nm gold) (C). In C, the small arrows point to caveolae containing PDGF receptors and the large arrows point to caveolae containing erk2. The asterisks indicate clathrin-coated pits. (Bars = 0.2 μm.)
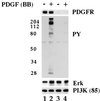
Figure 2
PDGF stimulates activation of caveolae MAP kinase in vivo. Normal human fibroblasts were incubated for the indicated time in the presence of PDGF(BB) at 30 ng/ml. At the end of the incubation, caveolae membranes and cytosol fractions were prepared. Caveolae membrane (5 μg per lane) and cytosol (25 μg per lane) were separated by SDS/PAGE on 10% gels and immunoblotted with either anti-activated MAP kinase IgG (AK) or anti-MAP kinase IgG (Erk).
Figure 3
PDGF stimulates tyrosine phosphorylation of caveolae proteins in vitro. Normal human fibroblasts were grown as described. Caveolae membranes were isolated from cells that had been incubated for 2 hr in the presence (lanes 3 and 4) or absence (lanes 1 and 2) of PDGF(BB) (40 ng/ml) and transferred to phosphorylation buffer. Aliquots of caveolae membranes (5 μg per tube) were incubated in the presence (lanes 2 and 4) or absence (lanes 1 and 3) of PDGF(BB) (30 ng/ml) for 30 min on ice followed by 5 min at 37°C. The reaction was stopped by adding ice-cold PBS. The samples were pelleted, dissolved in SDS sample buffer, and immunoblotted with the indicated antibody.
Figure 4
Immunogold localization of tyrosine-phosphorylated proteins. Normal human fibroblasts were grown. (A and B) One set of cells was incubated in the presence (B) or absence (A) of PDGF(BB) (30 ng/ml) for 15 min. Membranes were attached to grids and incubated with anti-phosphotyrosine IgG followed by anti-IgG conjugated to 10-nm gold. Small arrows point to caveolae membrane. Asterisks mark clathrin coated pits. (C and D) A second set of cells was used to isolate caveolae. Isolated caveolae were incubated in the presence (D) or absence (C) of PDGF(BB) (30 ng/ml) for 5 min before labeling with the anti-phosphotyrosine IgG followed by gold (10 nm)-conjugated second antibody. Small arrows point to isolated membrane. [Bars = 0.3 μm (A and B) and 0.4 μM (C and D).]
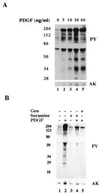
Figure 5
PDGF stimulates tyrosine phosphorylation and MAP kinase activation in vitro. Caveolae were isolated from normal human fibroblasts grown overnight in the absence of serum. (A) Aliquots of caveolae membranes (5 μg per tube) were incubated on ice for 30 min in the presence of the indicated concentration of PDGF(BB) followed by 5 min at 37°C. (B) Aliquots of caveolae membranes (5 μg per tube) were pretreated with either genistein (100 μg/ml; lane 5) or suramine (100 μg/ml; lanes 3 and 4) for 30 min on ice. Samples were then incubated in the presence (lanes 2, 4, and 5) or absence (lanes 1 and 3) of PDGF(BB) (100 ng/ml) for another 30 min on ice followed by 5 min at 37°C. All reactions were stopped by adding 7% trichloroacetic acid and then immunoblotted with either anti-phosphotyrosine IgG (PY) or anti-activated MAP kinase IgG (AK). The distortion in the gel (blank space) is due to the high concentration of BSA present in the phosphorylation buffer.
References
- Mellstrom K, Heldin C-H, Westermark B. Exp Cell Res. 1988;177:347–359. - PubMed
- Cochran B H, Reffel A C, Stiles C D. Cell. 1983;33:939–947. - PubMed
- Noble M, Murray K, Stroobant P, Waterfield M D, Riddle P. Nature (London) 1988;333:560–562. - PubMed
- Moolenaar W H, Tertooler L G J, de Last S W. J Biol Chem. 1984;259:8066–8069. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources