Induction of long-term depression and rebound potentiation by inositol trisphosphate in cerebellar Purkinje neurons - PubMed (original) (raw)
Induction of long-term depression and rebound potentiation by inositol trisphosphate in cerebellar Purkinje neurons
K Khodakhah et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Cerebellar Purkinje neurons receive two major excitatory inputs, the climbing fibers (CFs) and parallel fibers (PFs). Simultaneous, repeated activation of CFs and PFs results in the long-term depression (LTD) of the amplitude of PF-evoked synaptic currents. To induce LTD, activation of CFs may be substituted with depolarization of the Purkinje neuron to turn on voltage-activated calcium channels and increase the intracellular calcium concentration. The role of PFs in the induction of LTD, however, is less clear. PFs activate glutamate metabotropic receptors that increase phosphoinositide turnover and elevate cytosolic inositol 1,4,5-trisphosphate (InsP3). It has been proposed that calcium release from intracellular stores via InsP3 receptors may be important in the induction of LTD. We studied the role of InsP3 in the induction of LTD by photolytic release of InsP3 from its biologically inactive "caged" precursor in voltage-clamped Purkinje neurons in acutely prepared cerebellar slices. We find that InsP3-evoked calcium release is as effective in LTD induction as activation of PFs. InsP3-induced LTD was prevented by calcium chelator 1,2-bis(2-amino phenoxy)ethane-N,N,N', N'-tetraacetic acid. LTD produced either by repeated activation of PFs combined with depolarization (PF+DeltaV), or by InsP3 combined with depolarization (InsP3+DeltaV) saturated at approximately 50%. Maximal LTD induced by PF+DeltaV could not be further increased by InsP3+DeltaV and vice versa, which suggests that both protocols for induction of LTD share a common path. In addition to inducing LTD, photo-release of InsP3+DeltaV resulted in the rebound potentiation of inhibitory synaptic currents. In the presence of heparin, an InsP3 receptor antagonist, repeated activation of PF+DeltaV failed to induce LTD, suggesting that InsP3 receptors play an important role in LTD induction under physiological conditions.
Figures
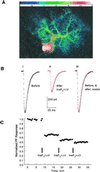
Figure 1
(A) The fluorescence emitted by the calcium indicator furaptra was integrated every 300 ms and calibrated to yield estimates of [Ca2+]i. Shown is [Ca2+]i (color calibration bar in μM) in a Purkinje neuron 50 ms after photo-release of 40 μM InsP3. Calcium is mobilized in the soma as well as in the extensive dendritic tree. (Bi) Representative PF-evoked excitatory synaptic current record in a voltage-clamped Purkinje neuron. (Bii) After a stable baseline, 40 μM InsP3 was photo-released in the cytosol with a 1-ms pulse of UV light. Fifty milliseconds after the flash the cell was depolarized to 0 mV for 50 ms. Stimulation of PFs after coincident InsP3+ΔV resulted in smaller inward currents. (Biii) Scaling of the PF-evoked synaptic currents after LTD to the same amplitude as that of control responses reveals that the kinetics of synaptic currents were not altered after LTD. Experiment JL245A. (C) Relative amplitudes of peak PF-evoked inward synaptic currents are plotted against time. Concurrent InsP3+ΔV, delivered at the time indicated by the arrow, reduced the amplitude of subsequent PF-evoked synaptic currents. Additional pulses of InsP3+ΔV resulted in much smaller reduction in the amplitude of the synaptic currents, although the InsP3-evoked calcium release was the same (data not shown). Experiment JN096A.
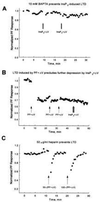
Figure 2
(A) Intracellular calcium buffering prevents LTD. A Purkinje neuron was voltage-clamped with a patch pipette filled with solution containing 10 mM 1,2-bis(2-amino phenoxy)ethane-N,N,_N_′,_N_′-tetraacetic acid. Two pulses of InsP3+ΔV (arrows) failed to induce LTD. Experiment JL255A. (B) Evidence of saturation. A 30-s train of PF+ΔV (arrow) was used to induce LTD. Two subsequent 30-s trains of PF+ΔV (second and third arrows) failed to induce additional depression as maximal depression was achieved with the first train. Photo-release of InsP3+ΔV (hashed arrow) also failed to induce additional depression, which suggests a common pathway for both protocols. (C) The InsP3 receptor antagonist heparin (50 μg/ml in the patch pipette) prevented induction of LTD by 30-s (first arrow) and 100-s (second arrow) trains of PF+ΔV, indicating that InsP3-evoked calcium release is required for induction of LTD under physiological conditions. The transient decreases in responses after the trains are because of increases in membrane conductance presumably caused by calcium-activated K+ and Cl− channels. In all other figures amplitude of synaptic currents are shown after return of the membrane conductance to its resting level. Experiment JL166A.
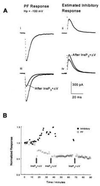
Figure 3
(Ai) PF-evoked excitatory synaptic currents were recorded by clamping a Purkinje cell at the chloride reversal potential, −100 mV. (Aii) The “pure” contribution of inhibitory synaptic currents were estimated from currents recorded at −50 mV as described in the text. (Aiii) Concurrent photo-release of 40 μM InsP3 together with depolarization of the Purkinje cell to 0 mV for 50 ms decreased the amplitude of the PF-evoked synaptic current, and (Aiv) facilitated the amplitude of the inhibitory synaptic current. Qualitatively similar results were observed in three other cells. Experiment JL265A, cell no. 2. (B) Time course of normalized amplitudes of the PF-evoked excitatory (○) and inhibitory (•) synaptic currents of the experiment in A. InsP3+ΔV depressed the PF responses while increasing the inhibitory synaptic current. Subsequent pulses of InsP3+ΔV did not induce more than 50% depression of the excitatory synaptic currents.
Similar articles
- Functional heterogeneity of calcium release by inositol trisphosphate in single Purkinje neurones, cultured cerebellar astrocytes, and peripheral tissues.
Khodakhah K, Ogden D. Khodakhah K, et al. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4976-80. doi: 10.1073/pnas.90.11.4976. Proc Natl Acad Sci U S A. 1993. PMID: 8506344 Free PMC article. - Fast activation and inactivation of inositol trisphosphate-evoked Ca2+ release in rat cerebellar Purkinje neurones.
Khodakhah K, Ogden D. Khodakhah K, et al. J Physiol. 1995 Sep 1;487 ( Pt 2)(Pt 2):343-58. doi: 10.1113/jphysiol.1995.sp020884. J Physiol. 1995. PMID: 8558468 Free PMC article. - Involvement of inositol trisphosphate in cerebellar long-term depression.
Kasono K, Hirano T. Kasono K, et al. Neuroreport. 1995 Feb 15;6(3):569-72. doi: 10.1097/00001756-199502000-00040. Neuroreport. 1995. PMID: 7539306 - Calcium requirement of long-term depression and rebound potentiation in cerebellar Purkinje neurons.
Tempia F, Konnerth A. Tempia F, et al. Semin Cell Biol. 1994 Aug;5(4):243-50. doi: 10.1006/scel.1994.1030. Semin Cell Biol. 1994. PMID: 7994008 Review. - Mechanisms of intracellular calcium release during hormone and neurotransmitter action investigated with flash photolysis.
Ogden DC, Khodakhah K, Carter TD, Gray PT, Capiod T. Ogden DC, et al. J Exp Biol. 1993 Nov;184:105-27. doi: 10.1242/jeb.184.1.105. J Exp Biol. 1993. PMID: 7903678 Review.
Cited by
- Impaired calcium release in cerebellar Purkinje neurons maintained in culture.
Womack MD, Walker JW, Khodakhah K. Womack MD, et al. J Gen Physiol. 2000 Mar;115(3):339-46. doi: 10.1085/jgp.115.3.339. J Gen Physiol. 2000. PMID: 10694261 Free PMC article. - Presynaptic Spike Timing-Dependent Long-Term Depression in the Mouse Hippocampus.
Andrade-Talavera Y, Duque-Feria P, Paulsen O, Rodríguez-Moreno A. Andrade-Talavera Y, et al. Cereb Cortex. 2016 Aug;26(8):3637-3654. doi: 10.1093/cercor/bhw172. Epub 2016 Jun 9. Cereb Cortex. 2016. PMID: 27282393 Free PMC article. - Endoplasmic reticulum-mediated signalling in cellular microdomains.
Biwer LA, Isakson BE. Biwer LA, et al. Acta Physiol (Oxf). 2017 Jan;219(1):162-175. doi: 10.1111/apha.12675. Epub 2016 Apr 5. Acta Physiol (Oxf). 2017. PMID: 26973141 Free PMC article. Review. - Purkinje cell long-term depression is prevented by T-588, a neuroprotective compound that reduces cytosolic calcium release from intracellular stores.
Kimura T, Sugimori M, Llinás RR. Kimura T, et al. Proc Natl Acad Sci U S A. 2005 Nov 22;102(47):17160-5. doi: 10.1073/pnas.0508190102. Epub 2005 Nov 8. Proc Natl Acad Sci U S A. 2005. PMID: 16278299 Free PMC article. - mGluR1/TRPC3-mediated Synaptic Transmission and Calcium Signaling in Mammalian Central Neurons.
Hartmann J, Henning HA, Konnerth A. Hartmann J, et al. Cold Spring Harb Perspect Biol. 2011 Apr 1;3(4):a006726. doi: 10.1101/cshperspect.a006726. Cold Spring Harb Perspect Biol. 2011. PMID: 21441586 Free PMC article. Review.
References
- Ito M. The Cerebellum and Neural Control. New York: Raven; 1984.
- Albus J S. Math Biosci. 1971;10:25–61.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous