Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson's disease in rats - PubMed (original) (raw)
Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson's disease in rats
R J Mandel et al. Proc Natl Acad Sci U S A. 1997.
Abstract
A recombinant adeno-associated virus (rAAV) vector capable of infecting cells and expressing rat glial cell line-derived neurotrophic factor (rGDNF), a putative central nervous system dopaminergic survival factor, under the control of a potent cytomegalovirus (CMV) immediate/early promoter (AAV-MD-rGDNF) was constructed. Two experiments were performed to evaluate the time course of expression of rAAV-mediated GDNF protein expression and to test the vector in an animal model of Parkinson's disease. To evaluate the ability of rAAV-rGDNF to protect nigral dopaminergic neurons in the progressive Sauer and Oertel 6-hydroxydopamine (6-OHDA) lesion model, rats received perinigral injections of either rAAV-rGDNF virus or rAAV-lacZ control virus 3 weeks prior to a striatal 6-OHDA lesion and were sacrificed 4 weeks after 6-OHDA. Cell counts of back-labeled fluorogold-positive neurons in the substantia nigra revealed that rAAV-MD-rGDNF protected a significant number of cells when compared with cell counts of rAAV-CMV-lacZ-injected rats (94% vs. 51%, respectively). In close agreement, 85% of tyrosine hydroxylase-positive cells remained in the nigral rAAV-MD-rGDNF group vs. only 49% in the lacZ group. A separate group of rats were given identical perinigral virus injections and were sacrificed at 3 and 10 weeks after surgery. Nigral GDNF protein expression remained relatively stable over the 10 weeks investigated. These data indicate that the use of rAAV, a noncytopathic viral vector, can promote delivery of functional levels of GDNF in a degenerative model of Parkinson's disease.
Figures
Figure 4
Photomicrographs of nigral β-galactosidase expression 7 weeks after rAAV-CMV-lacZ injection. The nigral sections shown were taken from an rAAV-CMV-_lacZ_-injected rat that showed no evidence of a 6-OHDA lesion, and the rat was removed from the study. rAAV-CMV-_lacZ_-injected animals that were included in the study were found to have little or no β-galactosidase staining, probably because of the destruction of transduced cells by the subsequent striatal 6-OHDA lesion. (A) Low-power micrograph of the SN. The arrow indicates the injection site. (Bar = 200 μm.) (B) Higher-power micrograph of the same field. The arrow indicates the injection site. As has been observed (24, 41) most of the cells expressing β-galactosidase appear to be neurons. (Bar = 100 μm.)
Figure 1
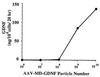
Production of GDNF in vitro from rAAV-MD-GDNF-transduced HeLa cells. Released GDNF levels were measured after addition of increasing amounts of rAAV-MD-GDNF vector to cultures containing 3 × 105 HeLa cells. The GDNF was measured by ELISA from samples taken after 20 hr from the culture medium.
Figure 2
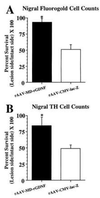
Nigral cell survival 4 weeks after a striatal 6-OHDA lesion. (A) Cell survival as estimated from cell counts of nigral FG+ cells. Nigral injection of rAAV-MD-rGDNF (solid bar, n = 6) resulted in a significantly greater number of FG+ cells surviving (indicated by *) after the striatal 6-OHDA lesion as compared with the rAAV-CMV-lacZ [open bar, n = 7, F(1, 11) = 20.1, P < 0.001]. (B) Cell survival estimated from cell counts of nigral TH+ cells. Nigral injection of rAAV-MD-rGDNF (solid bar, n = 6) resulted in a significantly greater number of TH+ cells surviving (indicated by *) after the striatal 6-OHDA lesion as compared with the rAAV-CMV-lacZ [open bar, n = 7, F(1, 11) = 6.9, P = 0.02]. Error bars represent +1 SEM.
Figure 3
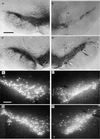
Photomicrographs of intact and lesioned/vector-injected SN 4 weeks after a striatal 6-OHDA lesion. (A and C) Photomicrographs of TH immunocytochemical staining of intact SN from a representative rAAV-CMV-_lacZ_-injected animal and a representative rAAV-MD-rGDNF-injected rat, respectively. (B and D) Photomicrographs of TH staining of the SN from lesioned and vector-injected hemisphere of representative rAAV-CMV-_lacZ_-injected animal and a representative rAAV-MD-rGDNF-injected rat, respectively. The arrows indicate a vector injection site. The cell loss due to the 6-OHDA lesion seen in B was significantly diminished (see Results) by pretreatment with rAAV-MD-rGDNF as demonstrated in D. The bar in A = 200 μm and applies to A–D. (E and G) Photomicrographs of FG back-labeled SN from the intact hemisphere from a representative rAAV-CMV-_lacZ_-injected animal and a representative rAAV-MD-rGDNF-injected rat, respectively. (F and H) Photomicrographs of FG back-labeled SN from the lesioned and vector-injected hemisphere from a representative rAAV-CMV-_lacZ_-injected animal and a representative rAAV-MD-rGDNF-injected rat, respectively. In addition to the 6-OHDA-induced cell loss demonstrated in F, there are numerous shrunken cells, punctae, and fibers with varicosities that are not apparent in the rAAV-MD-rGDNF-injected nigra shown in H. This pattern of 6-OHDA-induced damaged in FG back-labeled SN has been reported previously (17, 21, 22). The bar in E = 100 μm and applies to E–H.
Similar articles
- Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model.
Björklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C, Mandel RJ. Björklund A, et al. Brain Res. 2000 Dec 15;886(1-2):82-98. doi: 10.1016/s0006-8993(00)02915-2. Brain Res. 2000. PMID: 11119690 Review. - Astrocyte delivery of glial cell line-derived neurotrophic factor in a mouse model of Parkinson's disease.
Cunningham LA, Su C. Cunningham LA, et al. Exp Neurol. 2002 Apr;174(2):230-42. doi: 10.1006/exnr.2002.7877. Exp Neurol. 2002. PMID: 11922664 - Delayed delivery of AAV-GDNF prevents nigral neurodegeneration and promotes functional recovery in a rat model of Parkinson's disease.
Wang L, Muramatsu S, Lu Y, Ikeguchi K, Fujimoto K, Okada T, Mizukami H, Hanazono Y, Kume A, Urano F, Ichinose H, Nagatsu T, Nakano I, Ozawa K. Wang L, et al. Gene Ther. 2002 Mar;9(6):381-9. doi: 10.1038/sj.gt.3301682. Gene Ther. 2002. PMID: 11960314 - Adenoviral vector-mediated delivery of glial cell line-derived neurotrophic factor provides neuroprotection in the aged parkinsonian rat.
Connor B. Connor B. Clin Exp Pharmacol Physiol. 2001 Nov;28(11):896-900. doi: 10.1046/j.1440-1681.2001.03544.x. Clin Exp Pharmacol Physiol. 2001. PMID: 11703392 Review.
Cited by
- Glial cell line-derived neurotrophic factor partially ameliorates motor symptoms without slowing neurodegeneration in mice with respiratory chain-deficient dopamine neurons.
Sterky FH, Pernold K, Harvey BK, Lindqvist E, Hoffer BJ, Olson L. Sterky FH, et al. Cell Transplant. 2013;22(9):1529-39. doi: 10.3727/096368912X657693. Epub 2012 Oct 8. Cell Transplant. 2013. PMID: 23051605 Free PMC article. - A dual-hit animal model for age-related parkinsonism.
Boger HA, Granholm AC, McGinty JF, Middaugh LD. Boger HA, et al. Prog Neurobiol. 2010 Feb 9;90(2):217-29. doi: 10.1016/j.pneurobio.2009.10.013. Epub 2009 Oct 21. Prog Neurobiol. 2010. PMID: 19853012 Free PMC article. Review. - Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson's model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system.
Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. Kirik D, et al. J Neurosci. 2000 Jun 15;20(12):4686-700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. J Neurosci. 2000. PMID: 10844038 Free PMC article. - Self-complementary adeno-associated viral vectors for gene therapy of hemophilia B: progress and challenges.
Raj D, Davidoff AM, Nathwani AC. Raj D, et al. Expert Rev Hematol. 2011 Oct;4(5):539-49. doi: 10.1586/ehm.11.48. Expert Rev Hematol. 2011. PMID: 21939421 Free PMC article. Review.
References
- Hou J G G, Lin L F H, Mytilineou C. J Neurochem. 1996;66:74–82. - PubMed
- Clarkson E D, Zawada W M, Freed C R. Neuroreport. 1995;7:145–149. - PubMed
- Kearns C M, Gash D M. Brain Res. 1995;672:104–111. - PubMed
- Hoffer B J, Hoffman A, Bowenkamp K, Huettl P, Hudson J, Martin D, Lin L F H, Gerhardt G A. Neurosci Lett. 1994;182:107–111. - PubMed
- Shults C W, Kimber T, Martin D. Neuroreport. 1996;7:627–631. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical