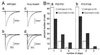Impaired motor coordination and persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking Galphaq - PubMed (original) (raw)
Impaired motor coordination and persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking Galphaq
S Offermanns et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Mice lacking the alpha-subunit of the heterotrimeric guanine nucleotide binding protein Gq (Galphaq) are viable but suffer from ataxia with typical signs of motor discoordination. The anatomy of the cerebellum is not overtly disturbed, and excitatory synaptic transmission from parallel fibers to cerebellar Purkinje cells (PCs) and from climbing fibers (CFs) to PCs is functional. However, about 40% of adult Galphaq mutant PCs remain multiply innervated by CFs because of a defect in regression of supernumerary CFs in the third postnatal week. Evidence is provided suggesting that Galphaq is part of a signaling pathway that is involved in the elimination of multiple CF innervation during this period.
Figures
Figure 1
Targeted disruption of the murine Gαq gene. (A) Part of the wild-type Gαq locus containing the two last exons (wt allele), the targeting construct, and the targeted locus (mut. allele) are shown. Neo, neomycin resistance gene. The sizes of the _Afl_II fragments predicted to hybridize to the indicated diagnostic probe are shown. Restriction endonucleases: A, _Afl_II; B, _Bgl_II; N, _Nde_I; S, _Sac_I; Sm, _Sma_I; and X, _Xho_I. (B) Southern blot analysis of _Afl_II-digested genomic DNA from wild-type (+/+), hemizygous (−/+), and homozygous mutant mice (−/−) with the diagnostic probe indicated in A. (C) Western blot analysis of whole brain cholate extracts from wild-type (+/+) and homozygous Gαq mutant mice (−/−) with antibodies recognizing the α-subunits of Gq (Gαq), G11 (Gα11), G13 (Gα13), and Go (Gαo). (D) Western blot analysis of cerebellar membrane fractions from wild-type (+/+) and homozygous Gαq mutant animals (−/−) with antibodies recognizing the α-subunits of Gq and G11 (Gαq/Gα11), Go (Gαo), type 1 metabotropic glutamate receptor (mGluR1), phospholipase C-β3 (PLC-β3), and phospholipase C-β4 (PLC-β4).
Figure 2
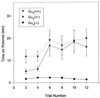
Rotorod test. Each point shows the mean ± SEM of the times each group stayed on the rod from each trail. n = 10 for Gαq (+/+) and Gαq (−/+); n = 12 for Gαq (−/−).
Figure 3
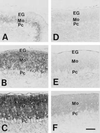
Immunohistochemistry for Gαq/Gα11 in the cerebellum of wild-type (A_–_C) and Gαq mutant mice (D_–_F). A and D, P7; B and E, P14; C and F, P21. EG, external granular layer; Pc, Purkinje cell layer. A_–_F were taken at the same magnification. [Bars = 50 μm (F).]
Figure 4
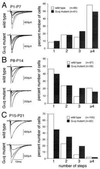
Multiple climbing fiber innervation of Purkinje cells in mature Gαq mutant mice. (A) EPSCs elicited by stimulation of climbing fibers (CFs) in the granule cell layer in wild-type (a, b) and Gαq mutant (c, d) Purkinje cells (PCs). Records in a, b, c, and d were taken from mice at postnatal day 50 (P50), P80, P46, and P46, respectively. One or two traces are superimposed at each threshold intensity. Stimuli were applied at 0.2 Hz. Holding potentials were −10 mV for a, b, and c and 0 mV for d. (B) Summary histograms showing number of discrete steps of CF-EPSCs of the wild-type (open columns) and mutant (hatched columns) PCs. Data obtained from mice at P22–P57 (a) and at P72–P109 (b), respectively. Numbers of tested PCs at P22–P57 (a) are: n = 115 (from seven wild-type mice) and n = 124 (from eight Gαq mutant mice). Those at P60–P109 (b) are: n = 72 (from four wild-type mice) and n = 42 (from three mice). All cells were studied blind to the mouse genotype.
Figure 5
Early postnatal development of CF innervation. (A Left) CF-EPSCs of the wild type (P7, holding potential, −30 mV) and Gαq mutant (P6, holding potential, −60 mV) PCs. One or two traces were superimposed at each threshold intensity. Stimuli were applied at 0.2 Hz. (A Right) Summary graph showing numbers of discrete steps of CF-EPSCs of the wild type (open columns) and mutant (hatched columns) PCs from mice at P1–P7. Numbers of tested PCs are n = 60 (from four wild-type mice, 45 cells studied blind to the mouse genotype) and n = 61 (from five Gαq mutant mice, 46 cells studied blind). (B) Similar to A, but with examples of CF-EPSCs of the wild type (P10, holding potential, −20 mV) and Gαq mutant (P10, holding potential, −20 mV) PCs and summary graph of the data from mice at P8–P14. Numbers of tested PCs are n = 97 (from six wild-type mice) and n = 58 (from four Gαq mutant mice). All cells were studied blind to the mouse genotype. (C) Similar to A and B, but with examples of CF-EPSCs of the wild type (P21, holding potential, −10 mV) and mutant (P18, holding potential, −10 mV) PCs and summary graph of the data from mice at P15–P21. Numbers of tested PCs are n = 105 (from six wild-type mice) and n = 62 (from four Gαq mutant mice). All cells were studied blind to the mouse genotype.
Similar articles
- Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGluR1.
Kano M, Hashimoto K, Kurihara H, Watanabe M, Inoue Y, Aiba A, Tonegawa S. Kano M, et al. Neuron. 1997 Jan;18(1):71-9. doi: 10.1016/s0896-6273(01)80047-7. Neuron. 1997. PMID: 9010206 - Roles of phospholipase Cbeta4 in synapse elimination and plasticity in developing and mature cerebellum.
Hashimoto K, Miyata M, Watanabe M, Kano M. Hashimoto K, et al. Mol Neurobiol. 2001 Feb;23(1):69-82. doi: 10.1385/MN:23:1:69. Mol Neurobiol. 2001. PMID: 11642544 Review. - Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice.
Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Aiba A, et al. Cell. 1994 Oct 21;79(2):377-88. Cell. 1994. PMID: 7954803 - Oscillating Purkinje neuron activity causing involuntary eye movement in a mutant mouse deficient in the glutamate receptor delta2 subunit.
Yoshida T, Katoh A, Ohtsuki G, Mishina M, Hirano T. Yoshida T, et al. J Neurosci. 2004 Mar 10;24(10):2440-8. doi: 10.1523/JNEUROSCI.0783-03.2004. J Neurosci. 2004. PMID: 15014119 Free PMC article. - Activity-dependent maturation of climbing fiber to Purkinje cell synapses during postnatal cerebellar development.
Kano M, Hashimoto K. Kano M, et al. Cerebellum. 2012 Jun;11(2):449-50. doi: 10.1007/s12311-011-0337-3. Cerebellum. 2012. PMID: 22194041 Review.
Cited by
- Direct and indirect pathways for heterosynaptic interaction underlying developmental synapse elimination in the mouse cerebellum.
Nakayama H, Miyazaki T, Abe M, Yamazaki M, Kawamura Y, Choo M, Konno K, Kawata S, Uesaka N, Hashimoto K, Miyata M, Sakimura K, Watanabe M, Kano M. Nakayama H, et al. Commun Biol. 2024 Jul 3;7(1):806. doi: 10.1038/s42003-024-06447-4. Commun Biol. 2024. PMID: 38961250 Free PMC article. - Capillary malformations.
Hammill AM, Boscolo E. Hammill AM, et al. J Clin Invest. 2024 Apr 15;134(8):e172842. doi: 10.1172/JCI172842. J Clin Invest. 2024. PMID: 38618955 Free PMC article. Review. - Regulation of cerebellar network development by granule cells and their molecules.
Kim M, Jun S, Park H, Tanaka-Yamamoto K, Yamamoto Y. Kim M, et al. Front Mol Neurosci. 2023 Jul 14;16:1236015. doi: 10.3389/fnmol.2023.1236015. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37520428 Free PMC article. Review. - Developmental expression of the Sturge-Weber syndrome-associated genetic mutation in Gnaq: a formal test of Happle's paradominant inheritance hypothesis.
Wetzel-Strong SE, Galeffi F, Benavides C, Patrucco M, Bullock JL, Gallione CJ, Lee HK, Marchuk DA. Wetzel-Strong SE, et al. Genetics. 2023 Aug 9;224(4):iyad077. doi: 10.1093/genetics/iyad077. Genetics. 2023. PMID: 37098137 Free PMC article. - Kazrin promotes dynein/dynactin-dependent traffic from early to recycling endosomes.
Hernandez-Perez I, Rubio J, Baumann A, Girao H, Ferrando M, Rebollo E, Aragay AM, Geli MI. Hernandez-Perez I, et al. Elife. 2023 Apr 25;12:e83793. doi: 10.7554/eLife.83793. Elife. 2023. PMID: 37096882 Free PMC article.
References
- Simon M I, Strathmann M P, Gautam N. Science. 1991;252:802–808. - PubMed
- Exton J H. Annu Rev Pharmacol Toxicol. 1996;36:481–509. - PubMed
- Wange R L, Smrcka A V, Sternweis P C, Exton J H. J Biol Chem. 1991;266:11409–11412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases