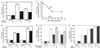Adenylyl cyclase 6 is selectively regulated by protein kinase A phosphorylation in a region involved in Galphas stimulation - PubMed (original) (raw)
Adenylyl cyclase 6 is selectively regulated by protein kinase A phosphorylation in a region involved in Galphas stimulation
Y Chen et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Receptors activate adenylyl cyclases through the Galphas subunit. Previous studies from our laboratory have shown in certain cell types that express adenylyl cyclase 6 (AC6), heterologous desensitization included reduction of the capability of adenylyl cyclases to be stimulated by Galphas. Here we further analyze protein kinase A (PKA) effects on adenylyl cyclases. PKA treatment of recombinant AC6 in insect cell membranes results in a selective loss of stimulation by high (>10 nM) concentrations of Galphas. Similar treatment of AC1 or AC2 did not affect Galphas stimulation. Conversion of Ser-674 in AC6 to an Ala blocks PKA phosphorylation and PKA-mediated loss of Galphas stimulation. A peptide encoding the region 660-682 of AC6 blocks stimulation of AC6 and AC2 by high concentrations of Galphas. Substitution of Ser-674 to Asp in the peptide renders the peptide ineffective, indicating that the region 660-682 of AC6 is involved in regulation of signal transfer from Galphas. This region contains a conserved motif present in most adenylyl cyclases; however, the PKA phosphorylation site is unique to members of the AC6 family. These observations suggest a mechanism of how isoform selective regulatory diversity can be obtained within conserved regions involved in signal communication.
Figures
Figure 1
Effect of PKA on adenylyl cyclase activities. Adenylyl cyclases were expressed in Hi-5 cells treated without or with PKA and then assayed for indicated activities. (A) Effect of PKA treatment on basal, 20 nM Q227L-Gαs (αs*), and 50 μM forskolin-stimulated AC6 activity. (B) Effect of PKA treatment on basal and 20 nM Q227L-Gαs (αs*) stimulated AC1 and AC2 activities. (C) Effect of varying periods of treatment with PKA on αs*-stimulated AC6 activity. (D) Effect of inclusion of 75 μM WIPTIDE during PKA treatment on basal, 20 nM Q227L-Gαs (αs*), and 50 μM forskolin-stimulated AC6 activity. (E) Effect of PKA treatment on Mn2+-stimulated AC6 activity. Divalent cation concentrations are in excess over 1 mM EDTA and 0.1 mM ATP.
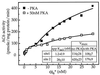
Figure 2
Effect of PKA treatment on stimulation of AC6 by varying concentrations of Gαs. AC6 containing Hi-5 cell membranes were treated in the presence and absence of PKA and then assayed in the presence of indicated concentrations of activated Gαs (αs*). Data were analyzed on a Sun Work station by using the program
prophet
. The plots of the data points and the fitted curves were generated by
prophet
. The data best fit a two-site model (8), and the indicated constants were obtained from the two site fit. The printed plots were exported to the
canvas
program in a Mac 8100 by use of an optical scanner. The plots were labeled within
canvas
and printed as
canvas
files.
Figure 3
Effect of mutating AC6 Ser-674 to Ala on PKA effects on AC6. (A) Protein kinase-mediated phosphorylation of Hi-5 cell membranes expressing wt and S674A forms of AC6. Treatment was in the absence or presence of 50 nM PKA or PKA and 75 μM WIPTIDE. After treatment the membranes were extracted and the FLAG-tagged AC6 and S674A-AC6 were isolated on anti FLAG-M2 columns. The column eluates were resolved by SDS/PAGE and visualized by autoradiography. For detailed procedures, see Materials and Methods. (B) Effect of PKA treatment of Hi-5 cell membranes expressing wt and S674A forms of AC6 on basal and activated Gαs (αs*)-stimulated activities. Treatment was in the absence or presence of PKA as described (7). Concentration of αs* in the assay was 20 nM.
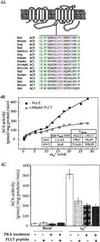
Figure 4
Effects of the peptide encoding region 660–682 of AC6 on Gαs regulation of adenylyl cyclases. (A) The region of AC6 containing the predicted PKA site used for the synthesis of the peptide is shown as part of the presumed topographical arrangement of mammalian adenylyl cyclases. Sequences of this region are compared between the different adenylyl cyclases from several species. Alignment of sequences was obtained by using the Genetics Computer Group program in a VAX computer. Fully conserved residues are shown in red. Mostly conserved charged residues are shown in blue, and mostly conserved hydrophobic residues are in green. The existence of a motif conserved amongst the different adenylyl cyclases is visually obvious. (B) AC6 containing Hi-5 cell membranes were assayed in the presence of indicated concentrations of activated Gαs (αs*) with or without the FLLT peptide. The data best fit a two-site model (8). Indicated constants were obtained from the two-site fit. (C) AC6 containing Hi-5 cell membranes that had been treated in the absence and presence of PKA were assayed for basal and 20 nM αs*-stimulated activities in the absence and presence of 300 μM FLLT peptide. (D) Comparison of the effect of the FLLT peptide and a modified peptide (S674D-FLLT peptide) where the Ser corresponding to Ser-674 was substituted with an Asp (D) on AC6 containing Hi-5 cell membrane basal and 20 nM αs*-stimulated adenylyl cyclase activities. Concentrations of both peptides were 300 μM. (E) Effect of varying concentrations of FLLT peptide on stimulation of AC6 by 17.5 nM αs*. (F) AC2 containing Hi-5 cell membranes assayed in the presence of indicated concentrations of αs* in the absence and presence of 300 μM FLLT peptide.
Figure 4
Effects of the peptide encoding region 660–682 of AC6 on Gαs regulation of adenylyl cyclases. (A) The region of AC6 containing the predicted PKA site used for the synthesis of the peptide is shown as part of the presumed topographical arrangement of mammalian adenylyl cyclases. Sequences of this region are compared between the different adenylyl cyclases from several species. Alignment of sequences was obtained by using the Genetics Computer Group program in a VAX computer. Fully conserved residues are shown in red. Mostly conserved charged residues are shown in blue, and mostly conserved hydrophobic residues are in green. The existence of a motif conserved amongst the different adenylyl cyclases is visually obvious. (B) AC6 containing Hi-5 cell membranes were assayed in the presence of indicated concentrations of activated Gαs (αs*) with or without the FLLT peptide. The data best fit a two-site model (8). Indicated constants were obtained from the two-site fit. (C) AC6 containing Hi-5 cell membranes that had been treated in the absence and presence of PKA were assayed for basal and 20 nM αs*-stimulated activities in the absence and presence of 300 μM FLLT peptide. (D) Comparison of the effect of the FLLT peptide and a modified peptide (S674D-FLLT peptide) where the Ser corresponding to Ser-674 was substituted with an Asp (D) on AC6 containing Hi-5 cell membrane basal and 20 nM αs*-stimulated adenylyl cyclase activities. Concentrations of both peptides were 300 μM. (E) Effect of varying concentrations of FLLT peptide on stimulation of AC6 by 17.5 nM αs*. (F) AC2 containing Hi-5 cell membranes assayed in the presence of indicated concentrations of αs* in the absence and presence of 300 μM FLLT peptide.
Similar articles
- Differential regulation of adenylyl cyclases by Galphas.
Harry A, Chen Y, Magnusson R, Iyengar R, Weng G. Harry A, et al. J Biol Chem. 1997 Jul 25;272(30):19017-21. doi: 10.1074/jbc.272.30.19017. J Biol Chem. 1997. PMID: 9228084 - Adenylyl cyclase isoform-selective regulation of vascular smooth muscle proliferation and cytoskeletal reorganization.
Gros R, Ding Q, Chorazyczewski J, Pickering JG, Limbird LE, Feldman RD. Gros R, et al. Circ Res. 2006 Oct 13;99(8):845-52. doi: 10.1161/01.RES.0000245189.21703.c0. Epub 2006 Sep 14. Circ Res. 2006. PMID: 16973907 - Complexity and diversity of mammalian adenylyl cyclases.
Sunahara RK, Dessauer CW, Gilman AG. Sunahara RK, et al. Annu Rev Pharmacol Toxicol. 1996;36:461-80. doi: 10.1146/annurev.pa.36.040196.002333. Annu Rev Pharmacol Toxicol. 1996. PMID: 8725398 Review. - The adenylyl cyclase family.
Krupinski J. Krupinski J. Mol Cell Biochem. 1991 May 29-Jun 12;104(1-2):73-9. doi: 10.1007/BF00229806. Mol Cell Biochem. 1991. PMID: 1656197 Review.
Cited by
- A key phosphorylation site in AC8 mediates regulation of Ca(2+)-dependent cAMP dynamics by an AC8-AKAP79-PKA signalling complex.
Willoughby D, Halls ML, Everett KL, Ciruela A, Skroblin P, Klussmann E, Cooper DM. Willoughby D, et al. J Cell Sci. 2012 Dec 1;125(Pt 23):5850-9. doi: 10.1242/jcs.111427. Epub 2012 Sep 12. J Cell Sci. 2012. PMID: 22976297 Free PMC article. - Physiological roles of mammalian transmembrane adenylyl cyclase isoforms.
Ostrom KF, LaVigne JE, Brust TF, Seifert R, Dessauer CW, Watts VJ, Ostrom RS. Ostrom KF, et al. Physiol Rev. 2022 Apr 1;102(2):815-857. doi: 10.1152/physrev.00013.2021. Epub 2021 Oct 26. Physiol Rev. 2022. PMID: 34698552 Free PMC article. Review. - Adenylyl cyclase isoforms 5 and 6 in the cardiovascular system: complex regulation and divergent roles.
Maghsoudi S, Shuaib R, Van Bastelaere B, Dakshinamurti S. Maghsoudi S, et al. Front Pharmacol. 2024 Apr 3;15:1370506. doi: 10.3389/fphar.2024.1370506. eCollection 2024. Front Pharmacol. 2024. PMID: 38633617 Free PMC article. Review. - International Union of Basic and Clinical Pharmacology. CI. Structures and Small Molecule Modulators of Mammalian Adenylyl Cyclases.
Dessauer CW, Watts VJ, Ostrom RS, Conti M, Dove S, Seifert R. Dessauer CW, et al. Pharmacol Rev. 2017 Apr;69(2):93-139. doi: 10.1124/pr.116.013078. Pharmacol Rev. 2017. PMID: 28255005 Free PMC article. Review. - Anti-inflammatory potential of CB1-mediated cAMP elevation in mast cells.
Small-Howard AL, Shimoda LM, Adra CN, Turner H. Small-Howard AL, et al. Biochem J. 2005 Jun 1;388(Pt 2):465-73. doi: 10.1042/BJ20041682. Biochem J. 2005. PMID: 15669919 Free PMC article.
References
- Gilman A G. Annu Rev Biochem. 1987;56:615–693. - PubMed
- Iyengar R. FASEB J. 1993;7:768–775. - PubMed
- Sunahara R K, Dessauer C W, Gilman A G. Annu Rev Pharmacol Toxicol. 1996;36:461–480. - PubMed
- Premont R T, Matsuoka I, Mattei M-G, Pouille Y, Defer N, Hanoune J. J Biol Chem. 1996;217:13900–13907. - PubMed
- Premont R T, Iyengar R. J Biol Chem. 1988;263:16087–16095. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK038761/DK/NIDDK NIH HHS/United States
- DK38761/DK/NIDDK NIH HHS/United States
- GM54508/GM/NIGMS NIH HHS/United States
- F31 GM015599/GM/NIGMS NIH HHS/United States
- DK-0745/DK/NIDDK NIH HHS/United States
- R01 GM054508/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases