The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination - PubMed (original) (raw)
The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination
S Einheber et al. J Cell Biol. 1997.
Abstract
We have investigated the potential role of contactin and contactin-associated protein (Caspr) in the axonal-glial interactions of myelination. In the nervous system, contactin is expressed by neurons, oligodendrocytes, and their progenitors, but not by Schwann cells. Expression of Caspr, a homologue of Neurexin IV, is restricted to neurons. Both contactin and Caspr are uniformly expressed at high levels on the surface of unensheathed neurites and are downregulated during myelination in vitro and in vivo. Contactin is downregulated along the entire myelinated nerve fiber. In contrast, Caspr expression initially remains elevated along segments of neurites associated with nascent myelin sheaths. With further maturation, Caspr is downregulated in the internode and becomes strikingly concentrated in the paranodal regions of the axon, suggesting that it redistributes from the internode to these sites. Caspr expression is similarly restricted to the paranodes of mature myelinated axons in the peripheral and central nervous systems; it is more diffusely and persistently expressed in gray matter and on unmyelinated axons. Immunoelectron microscopy demonstrated that Caspr is localized to the septate-like junctions that form between axons and the paranodal loops of myelinating cells. Caspr is poorly extracted by nonionic detergents, suggesting that it is associated with the axon cytoskeleton at these junctions. These results indicate that contactin and Caspr function independently during myelination and that their expression is regulated by glial ensheathment. They strongly implicate Caspr as a major transmembrane component of the paranodal junctions, whose molecular composition has previously been unknown, and suggest its role in the reciprocal signaling between axons and glia.
Figures
Figure 8
Schematic structure of the nodal region and the location of Caspr. The longitudinal organization of a myelinated axon at the node of Ranvier is shown. Myelinated axons contain three distinct domains: the internode, where the axon is surrounded by a compact myelin sheath; the paranodal region, where the axon is invaginated by and forms septate-like junctions with paranodal loops of the glial cell; and the node itself. The location of Caspr in the axon membrane is illustrated.
Figure 1
Expression of contactin and Caspr by neurons, Schwann cells and oligodendrocytes. Primary cultures of sensory neurons (A and B), Schwann cells (C and D), and oligodendrocytes (E and F) were stained with antibodies to contactin (A, C, and E) and Caspr (B, D, and F). Bar, 50 μm.
Figure 2
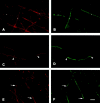
Contactin and Caspr expression in myelinating cocultures. Schwann cells were added to cultures of dissociated sensory neurons and allowed to repopulate the neurites. Ascorbic acid and serum were added to promote myelination, and cultures were fixed after an additional 3 wk and stained for contactin (A) and Caspr (C). Corresponding fields (B and D) were stained for MBP. Asterisks in A indicate an unensheathed fiber that expresses contactin at high levels. Arrows in C and D indicate nodes of Ranvier; arrowheads indicate isolated paranodes. The insets in C and D show the node in the center of the field at higher power. Bar, 50 μm.
Figure 3
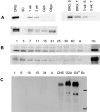
Immunoblot and Northern analysis of contactin and Caspr expression. (A) Caspr and contactin expression in cultured cells and association of Caspr with the detergent insoluble complex. 50 μg of protein lysate prepared from neuron (DRG), Schwann cell (SC), 1-wk-old, and 3-wk-old myelinating cocultures and 25 μg of lysate from oligodendrocyte progenitors (O-2A) and O4+ oligodendroglia (Oligo) were fractionated by SDS-PAGE, blotted onto nitrocellulose, and probed with antiserum specific for Caspr (top left) or contactin (bottom). The top right of A is an immunoblot of samples prepared from DRGs and 3-wk-old myelinating cultures extracted with Triton X-100 (T) and SDS (S) probed with the Caspr antiserum. (B) Changes in Caspr and contactin expression during postnatal sciatic nerve development. 100 μg of protein lysate, prepared from sciatic nerves of rats at postnatal days 1, 3, 7, 11, 15, 21, 25, 30, 40, and adult (A) were separated by SDS-PAGE, blotted onto nitrocellulose, and probed with either Caspr (B, top) or contactin (B, bottom) antisera. Note that the amounts of Caspr and contactin detected in sciatic nerve were considerably smaller than that in 100 μg of hippocampal lysate (Hc). (C) Northern blot analysis of contactin expression. A Northern blot with 10 μg per lane of total RNA isolated from postnatal day 1, 6, 10, 15, 34, and adult sciatic nerves, total rat brain (CNS) and cultured O-2A progenitors, O4+ oligodendroglia, and Schwann cells (Sc) was probed with a 32P-labeled human contactin cDNA probe. Bars indicate the position of 28S and 18S rRNA.
Figure 4
Redistribution of Caspr during myelination in vitro. Schwann cells were added to cultures of dissociated sensory neurons and allowed to repopulate the neurites. Ascorbic acid and serum were added to promote myelination, and cultures were fixed after an additional 4 d (A and B), 6 d (C and D), or 11 d (E and F). Cultures were immunostained for Caspr (A, C, and E) and MAG (B, D, and F). At 4 d, a few MAG-positive, nascent myelin segments were present (three are indicated with asterisks in B). Caspr expression continues to be expressed in the underlying axolemma (A, asterisks). 6 d after adding ascorbate, Caspr has begun to concentrate in some of the forming paranodes (C, arrowheads) associated with maturing myelin sheaths and, at the same time, is becoming attenuated in the corresponding internode. By 11 d, numerous myelin segments are present, and MAG staining in the Schmidt-Lanterman incisures is apparent (F). Caspr is concentrated in multiple paranodes and is substantially reduced in the internodes of mature myelin segments (located above the arrows in E), whereas it continues to be abundant in the internodes of nascent myelin segments (located below the arrows in E). Bar, 25 μm.
Figure 5
Caspr is concentrated in the paranodal regions of myelinated fibers in the PNS and CNS. A teased fiber preparation of adult sciatic nerve (A) and a coronal section through the facial nerve in the pons (B) were stained with an antiserum against Caspr using the immunoperoxidase technique and visualized by Nomarski (A) or brightfield microscopy (B). Low-magnification photomicrographs show that Caspr immunoreactivity is essentially restricted to the paranodes in both PNS and CNS fibers. Higher magnifications of individual nodes are shown in the insets. Bar, 20 μm.
Figure 6
Contactin/F3 is not concentrated in the paranodal region. Staining of the corpus callosum (cc) with antisera against Caspr and contactin/F3 is shown. Caspr staining (A) is concentrated in the paranodal regions of the small myelinated fibers of the corpus callosum (shown at higher magnification in the inset). Contactin/F3 immunoreactivity is concentrated in cells that may be interfascicular oligodendrocytes based on their morphology (arrows in the higher-magnification inset) and location. Minimal contactin/F3 immunoreactivity was apparent in the paranodal regions. Moderately intense but diffuse Caspr immunoreactivity in the lateral septal nucleus (SN in A) and light contactin/F3 staining of cells in the cortex (Ctx in B), some of which appear to be neurons, were also apparent. Bar, 100 μm.
Figure 7
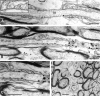
Immunoelectron microscopic localization of Caspr in myelinated and unmyelinated nerve fibers of the corpus callosum. Representative longitudinal sections of nodal regions of myelinated fibers with silver enhanced immunogold particles denoting the distribution of Caspr are shown in A–C. Gold particles were concentrated along the inner surface of the axonal membrane beneath the septate-like junctions located in the paranodal region (A–C). The inset in A shows the septate-like junctions that form between the axon and paranodal glial loops at higher magnification. Extremely dense labeling was observed where the plane of section approached the inner surface of the axonal membrane at the paranodes (pn; B and at higher magnification of the same field in C). Gold particles were occasionally found associated with the axonal membrane or cytoplasm in internodal portions of the myelinated fibers (B and C) but were rarely seen in the node (N). (D) A cross section demonstrating labeling of small caliber axons that are unmyelinated (u). Bars, 0.5 μm.
Similar articles
- Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination.
Rios JC, Melendez-Vasquez CV, Einheber S, Lustig M, Grumet M, Hemperly J, Peles E, Salzer JL. Rios JC, et al. J Neurosci. 2000 Nov 15;20(22):8354-64. doi: 10.1523/JNEUROSCI.20-22-08354.2000. J Neurosci. 2000. PMID: 11069942 Free PMC article. - An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axo-glial junction.
Tait S, Gunn-Moore F, Collinson JM, Huang J, Lubetzki C, Pedraza L, Sherman DL, Colman DR, Brophy PJ. Tait S, et al. J Cell Biol. 2000 Aug 7;150(3):657-66. doi: 10.1083/jcb.150.3.657. J Cell Biol. 2000. PMID: 10931875 Free PMC article. - Differential clustering of Caspr by oligodendrocytes and Schwann cells.
Eisenbach M, Kartvelishvily E, Eshed-Eisenbach Y, Watkins T, Sorensen A, Thomson C, Ranscht B, Barnett SC, Brophy P, Peles E. Eisenbach M, et al. J Neurosci Res. 2009 Nov 15;87(15):3492-501. doi: 10.1002/jnr.22157. J Neurosci Res. 2009. PMID: 19565653 - Neurexin IV, caspr and paranodin--novel members of the neurexin family: encounters of axons and glia.
Bellen HJ, Lu Y, Beckstead R, Bhat MA. Bellen HJ, et al. Trends Neurosci. 1998 Oct;21(10):444-9. doi: 10.1016/s0166-2236(98)01267-3. Trends Neurosci. 1998. PMID: 9786343 Review. - Molecular organization and function of vertebrate septate-like junctions.
Faivre-Sarrailh C. Faivre-Sarrailh C. Biochim Biophys Acta Biomembr. 2020 May 1;1862(5):183211. doi: 10.1016/j.bbamem.2020.183211. Epub 2020 Feb 4. Biochim Biophys Acta Biomembr. 2020. PMID: 32032590 Review.
Cited by
- Neurofascin 140 is an embryonic neuronal neurofascin isoform that promotes the assembly of the node of Ranvier.
Zhang A, Desmazieres A, Zonta B, Melrose S, Campbell G, Mahad D, Li Q, Sherman DL, Reynolds R, Brophy PJ. Zhang A, et al. J Neurosci. 2015 Feb 4;35(5):2246-54. doi: 10.1523/JNEUROSCI.3552-14.2015. J Neurosci. 2015. PMID: 25653379 Free PMC article. - No obvious abnormality in mice deficient in receptor protein tyrosine phosphatase beta.
Harroch S, Palmeri M, Rosenbluth J, Custer A, Okigaki M, Shrager P, Blum M, Buxbaum JD, Schlessinger J. Harroch S, et al. Mol Cell Biol. 2000 Oct;20(20):7706-15. doi: 10.1128/MCB.20.20.7706-7715.2000. Mol Cell Biol. 2000. PMID: 11003666 Free PMC article. - PGY repeats and N-glycans govern the trafficking of paranodin and its selective association with contactin and neurofascin-155.
Bonnon C, Bel C, Goutebroze L, Maigret B, Girault JA, Faivre-Sarrailh C. Bonnon C, et al. Mol Biol Cell. 2007 Jan;18(1):229-41. doi: 10.1091/mbc.e06-06-0570. Epub 2006 Nov 8. Mol Biol Cell. 2007. PMID: 17093057 Free PMC article. - Contactin associates with Na+ channels and increases their functional expression.
Kazarinova-Noyes K, Malhotra JD, McEwen DP, Mattei LN, Berglund EO, Ranscht B, Levinson SR, Schachner M, Shrager P, Isom LL, Xiao ZC. Kazarinova-Noyes K, et al. J Neurosci. 2001 Oct 1;21(19):7517-25. doi: 10.1523/JNEUROSCI.21-19-07517.2001. J Neurosci. 2001. PMID: 11567041 Free PMC article. - A myelin galactolipid, sulfatide, is essential for maintenance of ion channels on myelinated axon but not essential for initial cluster formation.
Ishibashi T, Dupree JL, Ikenaka K, Hirahara Y, Honke K, Peles E, Popko B, Suzuki K, Nishino H, Baba H. Ishibashi T, et al. J Neurosci. 2002 Aug 1;22(15):6507-14. doi: 10.1523/JNEUROSCI.22-15-06507.2002. J Neurosci. 2002. PMID: 12151530 Free PMC article.
References
- Andres KH. über die feinstruktur besonderer einrichtungen in markhaltigen nervenfasern des kleinhirns der ratte. Z Zellforsch. 1965;65:701–712. - PubMed
- Bargmann W, Lindner E. über den Feinbau des Nebennierenmarkes des Igels (Erinaceus europaeus L) . Z Zellforsch. 1964;64:868–912. - PubMed
- Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87:1059–1068. - PubMed
- Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. - PubMed
- Black, J.A., H. Sontheimer, Y. Oh, and S.G. Waxman. 1995. The oligodendrocyte, the perinodal astrocyte, and the central node of Ranvier. In The Axon. S. Waxman, J. Kocsis, and P. Stys, editors. Oxford University Press, New York. 116–143.
Publication types
MeSH terms
Substances
Grants and funding
- HL18974/HL/NHLBI NIH HHS/United States
- NS33165/NS/NINDS NIH HHS/United States
- P01 HL018974/HL/NHLBI NIH HHS/United States
- NS26001/NS/NINDS NIH HHS/United States
- R01 NS026001/NS/NINDS NIH HHS/United States
- T32 GM007308/GM/NIGMS NIH HHS/United States
- R01 DA008259/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases