The E1B 19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein that activates apoptosis - PubMed (original) (raw)
The E1B 19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein that activates apoptosis
G Chen et al. J Exp Med. 1997.
Abstract
Nip3 (nineteen kD interacting protein-3) is an E1B 19K and Bcl-2 binding protein of unknown function. Nip3 is detected as both a 60- and 30-kD protein in vivo and in vitro and exhibits strong homologous interaction in a yeast two-hybrid system indicating that it can homodimerize. Nip3 is expressed in mitochondria and a mutant (Nip3(163)) lacking the putative transmembrane domain and COOH terminus does not dimerize or localize to mitochondria. Transient transfection of epitope-tagged Nip3 in Rat-1 fibroblasts and MCF-7 breast carcinoma induces apoptosis within 12 h while cells transfected with the Nip3(163) mutant have a normal phenotype, suggesting that mitochondrial localization is necessary for induction of cell death. Nip3 overexpression increases the sensitivity to apoptosis induced by granzyme B and topoisomerase I and II inhibitors. After transfection, both Nip3 and Nip3(163) protein levels decrease steadily over 48 h indicating that the protein is rapidly degraded and this occurs in the absence of cell death. Bcl-2 overexpression initially delays the onset of apoptosis induced by Nip3 but the resistance is completely overcome in longer periods of incubation. Nip3 protein levels are much higher and persist longer in Bcl-2 expressing cells. In conclusion, Nip3 is an apoptosis-inducing dimeric mitochondrial protein that can overcome Bcl-2 suppression.
Figures
Figure 1
(A) Nip3 mRNA of 1535 bp is shown with the open reading frame boxed with the predicted protein of 194 amino acids. The putative transmembrane domain between amino acids 164 and 184 of Nip3 protein is shaded. A mutant lacking the transmembrane sequence was constructed by truncation at amino acid 163 and is called Nip3163. (B) Nip3 Northern blot of RNA extracted from multiple murine tissues as indicated, and human MCF-7 mammary carcinoma cells. Transcript sizes are noted on the right.
Figure 1
(A) Nip3 mRNA of 1535 bp is shown with the open reading frame boxed with the predicted protein of 194 amino acids. The putative transmembrane domain between amino acids 164 and 184 of Nip3 protein is shaded. A mutant lacking the transmembrane sequence was constructed by truncation at amino acid 163 and is called Nip3163. (B) Nip3 Northern blot of RNA extracted from multiple murine tissues as indicated, and human MCF-7 mammary carcinoma cells. Transcript sizes are noted on the right.
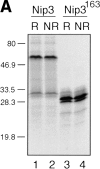
Figure 2
In vitro expression of Nip3 as a homodimer. (A) After in vitro transcription and translation of Nip3 the 35S-labeled protein was separated on SDS-PAGE (lanes 1 and 2) under reducing (R, lane 1) and nonreducing (NR, lane 2) conditions. Nip3 runs as two bands at 60 and 30 kD. Nip3163 is a truncated mutant in which the terminal 31 amino acids from 164 to 194 containing the putative transmembrane domain have been removed (lanes 3 and 4). The truncated Nip3163 is expressed as a major band at 29 kD and a minor band at 28 kD under both reducing (lane 3) and nonreducing (lane 4) conditions. (B) Comparative peptide mapping of in vitro translated 35S-labeled 60-kD Nip3 and 28-kD Nip3163 protein. After in vitro translation, Nip3 and Nip3163 were immunoprecipitated, the 60- and 28-kD bands recovered after SDS-PAGE then trypsin digested. The resulting peptides were separated on the same plate by electrophoresis at pH 1.9 (horizontal dimension with anode to the left), followed by ascending chromatography. The positions of the origin of Nip3 and Nip3163 are marked by arrows. Three [35S]methionine-labeled peptides predicted from the trypsin digest are circled and labeled A, B, and C (Nip3) and _A_′, _B_′, and _C_′ (Nip3163). Peptide A and A′, amino acids 1–45; B and B′, amino acids 140–146; C and C′, 147–152. All three peptides are represented in both Nip3 and Nip3163. Minor spots are similar in the two proteins and likely are partially digested fragments. One of two experiments with similar results is shown. (C) Yeast two-hybrid system identifies Nip3 homodimerization. (left) Plasmids encoding full-length Nip3 and the COOH-terminal truncated mutant Nip3163 fused to the GAL4 DNA-binding domain were cotransformed with plasmids encoding Nip3, Nip3163 or empty vector sequences fused to the GAL4 transcriptional activation domain. Protein–protein interactions were determined by growth of yeast in the absence of leucine, tryptophan and histidine. (Right) Growth in the absence of leucine and tryptophan is shown as a control. The results are representative of 3 independent experiments.
Figure 2
In vitro expression of Nip3 as a homodimer. (A) After in vitro transcription and translation of Nip3 the 35S-labeled protein was separated on SDS-PAGE (lanes 1 and 2) under reducing (R, lane 1) and nonreducing (NR, lane 2) conditions. Nip3 runs as two bands at 60 and 30 kD. Nip3163 is a truncated mutant in which the terminal 31 amino acids from 164 to 194 containing the putative transmembrane domain have been removed (lanes 3 and 4). The truncated Nip3163 is expressed as a major band at 29 kD and a minor band at 28 kD under both reducing (lane 3) and nonreducing (lane 4) conditions. (B) Comparative peptide mapping of in vitro translated 35S-labeled 60-kD Nip3 and 28-kD Nip3163 protein. After in vitro translation, Nip3 and Nip3163 were immunoprecipitated, the 60- and 28-kD bands recovered after SDS-PAGE then trypsin digested. The resulting peptides were separated on the same plate by electrophoresis at pH 1.9 (horizontal dimension with anode to the left), followed by ascending chromatography. The positions of the origin of Nip3 and Nip3163 are marked by arrows. Three [35S]methionine-labeled peptides predicted from the trypsin digest are circled and labeled A, B, and C (Nip3) and _A_′, _B_′, and _C_′ (Nip3163). Peptide A and A′, amino acids 1–45; B and B′, amino acids 140–146; C and C′, 147–152. All three peptides are represented in both Nip3 and Nip3163. Minor spots are similar in the two proteins and likely are partially digested fragments. One of two experiments with similar results is shown. (C) Yeast two-hybrid system identifies Nip3 homodimerization. (left) Plasmids encoding full-length Nip3 and the COOH-terminal truncated mutant Nip3163 fused to the GAL4 DNA-binding domain were cotransformed with plasmids encoding Nip3, Nip3163 or empty vector sequences fused to the GAL4 transcriptional activation domain. Protein–protein interactions were determined by growth of yeast in the absence of leucine, tryptophan and histidine. (Right) Growth in the absence of leucine and tryptophan is shown as a control. The results are representative of 3 independent experiments.
Figure 2
In vitro expression of Nip3 as a homodimer. (A) After in vitro transcription and translation of Nip3 the 35S-labeled protein was separated on SDS-PAGE (lanes 1 and 2) under reducing (R, lane 1) and nonreducing (NR, lane 2) conditions. Nip3 runs as two bands at 60 and 30 kD. Nip3163 is a truncated mutant in which the terminal 31 amino acids from 164 to 194 containing the putative transmembrane domain have been removed (lanes 3 and 4). The truncated Nip3163 is expressed as a major band at 29 kD and a minor band at 28 kD under both reducing (lane 3) and nonreducing (lane 4) conditions. (B) Comparative peptide mapping of in vitro translated 35S-labeled 60-kD Nip3 and 28-kD Nip3163 protein. After in vitro translation, Nip3 and Nip3163 were immunoprecipitated, the 60- and 28-kD bands recovered after SDS-PAGE then trypsin digested. The resulting peptides were separated on the same plate by electrophoresis at pH 1.9 (horizontal dimension with anode to the left), followed by ascending chromatography. The positions of the origin of Nip3 and Nip3163 are marked by arrows. Three [35S]methionine-labeled peptides predicted from the trypsin digest are circled and labeled A, B, and C (Nip3) and _A_′, _B_′, and _C_′ (Nip3163). Peptide A and A′, amino acids 1–45; B and B′, amino acids 140–146; C and C′, 147–152. All three peptides are represented in both Nip3 and Nip3163. Minor spots are similar in the two proteins and likely are partially digested fragments. One of two experiments with similar results is shown. (C) Yeast two-hybrid system identifies Nip3 homodimerization. (left) Plasmids encoding full-length Nip3 and the COOH-terminal truncated mutant Nip3163 fused to the GAL4 DNA-binding domain were cotransformed with plasmids encoding Nip3, Nip3163 or empty vector sequences fused to the GAL4 transcriptional activation domain. Protein–protein interactions were determined by growth of yeast in the absence of leucine, tryptophan and histidine. (Right) Growth in the absence of leucine and tryptophan is shown as a control. The results are representative of 3 independent experiments.
Figure 3
Subcellular localization of Nip3 and Nip3163. Rat-1 cells were transfected with HA-Nip3 and stained with anti-HA antibody using FITC (A) and the mitochondrial protein marker anti-HSP60 antibody using Cy3 (B). The stained images were combined to compare the staining pattern of both proteins (C) and their coincidence is indicated by the conversion of green FITC and red Cy3 stain to yellow thoughout the cell cytoplasm. HA-Nip3163 was expressed in Rat-1 cells then stained with anti-HA antibody (D), or anti-HSP60 antibody (E) and shown as a combined image using antibodies (F), as described above.
Figure 3
Subcellular localization of Nip3 and Nip3163. Rat-1 cells were transfected with HA-Nip3 and stained with anti-HA antibody using FITC (A) and the mitochondrial protein marker anti-HSP60 antibody using Cy3 (B). The stained images were combined to compare the staining pattern of both proteins (C) and their coincidence is indicated by the conversion of green FITC and red Cy3 stain to yellow thoughout the cell cytoplasm. HA-Nip3163 was expressed in Rat-1 cells then stained with anti-HA antibody (D), or anti-HSP60 antibody (E) and shown as a combined image using antibodies (F), as described above.
Figure 3
Subcellular localization of Nip3 and Nip3163. Rat-1 cells were transfected with HA-Nip3 and stained with anti-HA antibody using FITC (A) and the mitochondrial protein marker anti-HSP60 antibody using Cy3 (B). The stained images were combined to compare the staining pattern of both proteins (C) and their coincidence is indicated by the conversion of green FITC and red Cy3 stain to yellow thoughout the cell cytoplasm. HA-Nip3163 was expressed in Rat-1 cells then stained with anti-HA antibody (D), or anti-HSP60 antibody (E) and shown as a combined image using antibodies (F), as described above.
Figure 3
Subcellular localization of Nip3 and Nip3163. Rat-1 cells were transfected with HA-Nip3 and stained with anti-HA antibody using FITC (A) and the mitochondrial protein marker anti-HSP60 antibody using Cy3 (B). The stained images were combined to compare the staining pattern of both proteins (C) and their coincidence is indicated by the conversion of green FITC and red Cy3 stain to yellow thoughout the cell cytoplasm. HA-Nip3163 was expressed in Rat-1 cells then stained with anti-HA antibody (D), or anti-HSP60 antibody (E) and shown as a combined image using antibodies (F), as described above.
Figure 3
Subcellular localization of Nip3 and Nip3163. Rat-1 cells were transfected with HA-Nip3 and stained with anti-HA antibody using FITC (A) and the mitochondrial protein marker anti-HSP60 antibody using Cy3 (B). The stained images were combined to compare the staining pattern of both proteins (C) and their coincidence is indicated by the conversion of green FITC and red Cy3 stain to yellow thoughout the cell cytoplasm. HA-Nip3163 was expressed in Rat-1 cells then stained with anti-HA antibody (D), or anti-HSP60 antibody (E) and shown as a combined image using antibodies (F), as described above.
Figure 3
Subcellular localization of Nip3 and Nip3163. Rat-1 cells were transfected with HA-Nip3 and stained with anti-HA antibody using FITC (A) and the mitochondrial protein marker anti-HSP60 antibody using Cy3 (B). The stained images were combined to compare the staining pattern of both proteins (C) and their coincidence is indicated by the conversion of green FITC and red Cy3 stain to yellow thoughout the cell cytoplasm. HA-Nip3163 was expressed in Rat-1 cells then stained with anti-HA antibody (D), or anti-HSP60 antibody (E) and shown as a combined image using antibodies (F), as described above.
Figure 4
Overexpression of Nip3 but not Nip3163 induces apoptosis. (A) Rat-1 or MCF-7 (left) cells were transfected with T7-tagged Nip3 then at different times cells harvested and stained with anti-T7 antibody and FITC anti–mouse IgG antibody to identify cells expressing Nip3. The frequency of apoptotic cells was quantitated by Hoechst dye staining. Rat-1 cells (right) were then transfected and apoptotic cells expressing (•) and not expressing (○) Nip3 were quantitated. Rat-1 cells expressing (▾) or not expressing (▿) Nip3163 were analysed in the same manner. At least 200 cells were counted in each sample. All assays were repeated three to seven times with identical results. (B) Western blot of Rat-1 cells transfected with T7-Nip3 (left) or T7-Nip3163 (right). Cells were harvested at the times indicated and Western blots developed with anti-T7 antibody.
Figure 4
Overexpression of Nip3 but not Nip3163 induces apoptosis. (A) Rat-1 or MCF-7 (left) cells were transfected with T7-tagged Nip3 then at different times cells harvested and stained with anti-T7 antibody and FITC anti–mouse IgG antibody to identify cells expressing Nip3. The frequency of apoptotic cells was quantitated by Hoechst dye staining. Rat-1 cells (right) were then transfected and apoptotic cells expressing (•) and not expressing (○) Nip3 were quantitated. Rat-1 cells expressing (▾) or not expressing (▿) Nip3163 were analysed in the same manner. At least 200 cells were counted in each sample. All assays were repeated three to seven times with identical results. (B) Western blot of Rat-1 cells transfected with T7-Nip3 (left) or T7-Nip3163 (right). Cells were harvested at the times indicated and Western blots developed with anti-T7 antibody.
Figure 5
Nip3 sensitizes Rat-1 cells to drug-induced apoptosis. Rat-1 cells were transfected with T7-tagged Nip3 then 12 h later treated with increasing amounts of etoposide, camptothecin or granzyme B and perforin. Cells expressing Nip3 were identified by anti-T7 antibody and the nucleus stained with Hoechst dye. Apoptotic cells expressing Nip3 (Nip3 +) or cells not expressing Nip3 (Nip3 −) were enumerated. This is representative of three experiments showing similar results.
Figure 6
Nip3 overcomes Bcl-2 suppression of apoptosis. (A) (Left) Rat-1 and Rat-1/Bcl-2 cells were transfected with T7-Nip3 and at increasing time the cells were harvested and Nip3-expressing apoptotic cells quantitated as described in Fig. 4. (Right) The Rat-1 and Rat-1/ Bcl-2 cells were treated with granzyme B as shown at a constant concentration of perforin (125 ng/ml) for 3 h and apoptotic cells quantitated by Hoechst dye as described previously (22). Three other experiments showed similar results. (B) After transfection of Rat-1 and Rat-1/ Bcl-2 cells with T7-Nip3, lysates were harvested at increasing time intervals then Western blotted with anti-T7 antibody. Molecular mass markers are shown on the left. (C) Western blot of Bcl-2 (arrow) in Rat-1 (lane 1) and Rat-1/Bcl-2 (lane 2) cell lines. Blots were developed with rabbit anti–human Bcl-2 (PharMingen). Relative molecular mass (kD) is shown on the left.
Figure 6
Nip3 overcomes Bcl-2 suppression of apoptosis. (A) (Left) Rat-1 and Rat-1/Bcl-2 cells were transfected with T7-Nip3 and at increasing time the cells were harvested and Nip3-expressing apoptotic cells quantitated as described in Fig. 4. (Right) The Rat-1 and Rat-1/ Bcl-2 cells were treated with granzyme B as shown at a constant concentration of perforin (125 ng/ml) for 3 h and apoptotic cells quantitated by Hoechst dye as described previously (22). Three other experiments showed similar results. (B) After transfection of Rat-1 and Rat-1/ Bcl-2 cells with T7-Nip3, lysates were harvested at increasing time intervals then Western blotted with anti-T7 antibody. Molecular mass markers are shown on the left. (C) Western blot of Bcl-2 (arrow) in Rat-1 (lane 1) and Rat-1/Bcl-2 (lane 2) cell lines. Blots were developed with rabbit anti–human Bcl-2 (PharMingen). Relative molecular mass (kD) is shown on the left.
Figure 6
Nip3 overcomes Bcl-2 suppression of apoptosis. (A) (Left) Rat-1 and Rat-1/Bcl-2 cells were transfected with T7-Nip3 and at increasing time the cells were harvested and Nip3-expressing apoptotic cells quantitated as described in Fig. 4. (Right) The Rat-1 and Rat-1/ Bcl-2 cells were treated with granzyme B as shown at a constant concentration of perforin (125 ng/ml) for 3 h and apoptotic cells quantitated by Hoechst dye as described previously (22). Three other experiments showed similar results. (B) After transfection of Rat-1 and Rat-1/ Bcl-2 cells with T7-Nip3, lysates were harvested at increasing time intervals then Western blotted with anti-T7 antibody. Molecular mass markers are shown on the left. (C) Western blot of Bcl-2 (arrow) in Rat-1 (lane 1) and Rat-1/Bcl-2 (lane 2) cell lines. Blots were developed with rabbit anti–human Bcl-2 (PharMingen). Relative molecular mass (kD) is shown on the left.
Similar articles
- Adenovirus E1B-19K/BCL-2 interacting protein BNIP3 contains a BH3 domain and a mitochondrial targeting sequence.
Yasuda M, Theodorakis P, Subramanian T, Chinnadurai G. Yasuda M, et al. J Biol Chem. 1998 May 15;273(20):12415-21. doi: 10.1074/jbc.273.20.12415. J Biol Chem. 1998. PMID: 9575197 - A novel adenovirus E1B19K-binding protein B5 inhibits apoptosis induced by Nip3 by forming a heterodimer through the C-terminal hydrophobic region.
Ohi N, Tokunaga A, Tsunoda H, Nakano K, Haraguchi K, Oda K, Motoyama N, Nakajima T. Ohi N, et al. Cell Death Differ. 1999 Apr;6(4):314-25. doi: 10.1038/sj.cdd.4400493. Cell Death Differ. 1999. PMID: 10381623 - Nix and Nip3 form a subfamily of pro-apoptotic mitochondrial proteins.
Chen G, Cizeau J, Vande Velde C, Park JH, Bozek G, Bolton J, Shi L, Dubik D, Greenberg A. Chen G, et al. J Biol Chem. 1999 Jan 1;274(1):7-10. doi: 10.1074/jbc.274.1.7. J Biol Chem. 1999. PMID: 9867803 - Bcl-2/E1B 19 kDa-interacting protein 3-like protein (Bnip3L) interacts with bcl-2/Bcl-xL and induces apoptosis by altering mitochondrial membrane permeability.
Imazu T, Shimizu S, Tagami S, Matsushima M, Nakamura Y, Miki T, Okuyama A, Tsujimoto Y. Imazu T, et al. Oncogene. 1999 Aug 12;18(32):4523-9. doi: 10.1038/sj.onc.1202722. Oncogene. 1999. PMID: 10467396 - Viral homologs of BCL-2: role of apoptosis in the regulation of virus infection.
Cuconati A, White E. Cuconati A, et al. Genes Dev. 2002 Oct 1;16(19):2465-78. doi: 10.1101/gad.1012702. Genes Dev. 2002. PMID: 12368257 Review. No abstract available.
Cited by
- Identification of the hypoxia-inducible factor 1 alpha-responsive HGTD-P gene as a mediator in the mitochondrial apoptotic pathway.
Lee MJ, Kim JY, Suk K, Park JH. Lee MJ, et al. Mol Cell Biol. 2004 May;24(9):3918-27. doi: 10.1128/MCB.24.9.3918-3927.2004. Mol Cell Biol. 2004. PMID: 15082785 Free PMC article. - Overexpression of BH3-Only Protein BNIP3 Leads to Enhanced Tumor Growth.
Vijayalingam S, Pillai SG, Rashmi R, Subramanian T, Sagartz JE, Chinnadurai G. Vijayalingam S, et al. Genes Cancer. 2010 Sep;1(9):964-71. doi: 10.1177/1947601910386110. Genes Cancer. 2010. PMID: 21779475 Free PMC article. - Bnip3 Binds and Activates p300: Possible Role in Cardiac Transcription and Myocyte Morphology.
Thompson JW, Wei J, Appau K, Wang H, Yu H, Spiga MG, Graham RM, Webster KA. Thompson JW, et al. PLoS One. 2015 Aug 28;10(8):e0136847. doi: 10.1371/journal.pone.0136847. eCollection 2015. PLoS One. 2015. PMID: 26317696 Free PMC article. - BNIP3 subfamily BH3-only proteins: mitochondrial stress sensors in normal and pathological functions.
Chinnadurai G, Vijayalingam S, Gibson SB. Chinnadurai G, et al. Oncogene. 2008 Dec;27 Suppl 1(Suppl 1):S114-27. doi: 10.1038/onc.2009.49. Oncogene. 2008. PMID: 19641497 Free PMC article. Review. - Mitophagy: mechanisms, pathophysiological roles, and analysis.
Ding WX, Yin XM. Ding WX, et al. Biol Chem. 2012 Jul;393(7):547-64. doi: 10.1515/hsz-2012-0119. Biol Chem. 2012. PMID: 22944659 Free PMC article. Review.
References
- White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15. - PubMed
- Chinnaiyan AM, Dixit VM. The cell-death machine. Curr Biol. 1996;6:555–562. - PubMed
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. - PubMed
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. - PubMed
- Oltvai ZN, Korsmeyer SJ. Checkpoints of dueling dimers foil death wishes. Cell. 1994;79:189–192. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases