Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms - PubMed (original) (raw)
Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms
B Lemaitre et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Insects respond to microbial infection by the rapid and transient expression of several genes encoding potent antimicrobial peptides. Herein we demonstrate that this antimicrobial response of Drosophila is not aspecific but can discriminate between various classes of microorganisms. We first observe that the genes encoding antibacterial and antifungal peptides are differentially expressed after injection of distinct microorganisms. More strikingly, Drosophila that are naturally infected by entomopathogenic fungi exhibit an adapted response by producing only peptides with antifungal activities. This response is mediated through the selective activation of the Toll pathway.
Figures
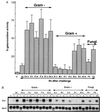
Figure 1
Induction of the diptericin gene in Drosophila adults infected by various microorganisms. (A) Drosophila adults (3–5 days old) carrying the Dipt-lacZ reporter gene (14) were pricked with a sterile needle dipped into culture pellets of distinct bacterial strains (OD of the pellet = 100), fungal spores (1010 spores per ml), or hyphae from various fungi. β-Galactosidase activity was measured 6 h after challenge at 25°C. Each bar represents the mean of several independent measurements with confidence interval (P < 5%). (B) Northern blot of total RNA extracted from one and six bacteria- or fungi-challenged wild-type (OregonR) male adults. The blot was successively hybridized with diptericin (Dipt) and ribosomal protein rp49 (Rp49) cDNA. Rp49 was used as an internal control for quantification of RNA. Conditions were as in A. C (control), unchallenged flies; Inj, simple injury; En.c., Enterobacter cloacae (−); S.t., Salmonella typhimurium (−); S.m., Serratia marcescens (Db1140 strain); P.a., Pseudomonas aeruginosas (−); Er.c., Erwinia carotovora; Es. c., E. coli (−); A.v., Aerococcus viridans (+); M.l., M. luteus (+); S.f., Streptococcus faecalis (+); S.a., Staphylococcus aureus (+); B.s., Bacillus subtilis (+); B.t., Bacillus thuringiensis (+) B.m., Bacillus megaterium (+); Fh, hyphal bodies; Fs, fungal spores from a mixture of Fusarium oxysporum, Neurospora crassa, and Botrytis cinerea.
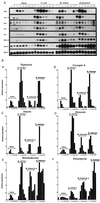
Figure 2
Time-course analysis of antimicrobial gene expression after infection by various microorganisms. (A) Northern blot of total RNA extracted from female wild-type (OregonR) adults at different time intervals after challenge (as indicated). Adult flies infected by pricking under the same conditions were kept at 29°C. The blot was successively hybridized with the following cDNA probes: diptericin (Dipt), cecropin A1 (Cec A), attacin (Att), drosocin (Drc), defensin (Def), metchnikowin (Metch), drosomycin (Drom), and rp49 (Rp49). Unchallenged females show a low level of drosomycin gene expression due to the constitutive expression in the sperm storage structures (D. Ferrandon, personal communication). This experiment was repeated several times and yielded similar results. (B) The signals on Northern blots of Fig. 3_A_ were quantified by a Bioimager system. The values were normalized with the corresponding value of rp49. The highest level of expression in a series was normalized as 100, and the results are given in relative activity (percent). Results obtained for defensin were similar to those for diptericin (data not shown).
Figure 3
Induction of antimicrobial peptide genes in fungi-infected adults. (A) Northern blot analysis of total RNA extracted from wild-type, _Tl_−, and Tl D mutant female adults. Flies were anesthetized and covered with spores of B. bassiana. Flies were placed at 29°C and collected after different time intervals. (B) Northern blot analysis of total RNA extracted from wild-type female adults after natural infection by various fungi. Flies were placed at 29°C and collected 5 days later. A and B were obtained separately. C, control; d, days; _Tl_−, Tl 632/Tl 1-RXA female adults; Tl D, unchallenged Tl 10b female adults; S.i. (septic injury) adults that were challenged by pricking with a needle dipped into a mixture of E. coli and M. luteus and collected 6 h later. B.b., B. bassiana; P.f., Paecylomyces fumoroseus; A.f., A. fumigatus; M.a., Metharizium anasiplae.
Similar articles
- Immune genes and divergent antimicrobial peptides in flies of the subgenus Drosophila.
Hanson MA, Hamilton PT, Perlman SJ. Hanson MA, et al. BMC Evol Biol. 2016 Oct 24;16(1):228. doi: 10.1186/s12862-016-0805-y. BMC Evol Biol. 2016. PMID: 27776480 Free PMC article. - The humoral antibacterial response of Drosophila.
Hoffmann JA, Hetru C, Reichhart JM. Hoffmann JA, et al. FEBS Lett. 1993 Jun 28;325(1-2):63-6. doi: 10.1016/0014-5793(93)81414-u. FEBS Lett. 1993. PMID: 8513894 Review. - Induction and regulation of antimicrobial peptides in Drosophila.
Engström Y. Engström Y. Dev Comp Immunol. 1999 Jun-Jul;23(4-5):345-58. doi: 10.1016/s0145-305x(99)00016-6. Dev Comp Immunol. 1999. PMID: 10426427 Review. - Peptidoglycan recognition protein (PGRP)-LE and PGRP-LC act synergistically in Drosophila immunity.
Takehana A, Yano T, Mita S, Kotani A, Oshima Y, Kurata S. Takehana A, et al. EMBO J. 2004 Nov 24;23(23):4690-700. doi: 10.1038/sj.emboj.7600466. Epub 2004 Nov 11. EMBO J. 2004. PMID: 15538387 Free PMC article. - A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense.
Lemaitre B, Kromer-Metzger E, Michaut L, Nicolas E, Meister M, Georgel P, Reichhart JM, Hoffmann JA. Lemaitre B, et al. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9465-9. doi: 10.1073/pnas.92.21.9465. Proc Natl Acad Sci U S A. 1995. PMID: 7568155 Free PMC article.
Cited by
- Drosophila host model reveals new enterococcus faecalis quorum-sensing associated virulence factors.
Teixeira N, Varahan S, Gorman MJ, Palmer KL, Zaidman-Remy A, Yokohata R, Nakayama J, Hancock LE, Jacinto A, Gilmore MS, de Fátima Silva Lopes M. Teixeira N, et al. PLoS One. 2013 May 29;8(5):e64740. doi: 10.1371/journal.pone.0064740. Print 2013. PLoS One. 2013. PMID: 23734216 Free PMC article. - Down syndrome cell adhesion molecule 1: testing for a role in insect immunity, behaviour and reproduction.
Peuß R, Wensing KU, Woestmann L, Eggert H, Milutinović B, Sroka MG, Scharsack JP, Kurtz J, Armitage SA. Peuß R, et al. R Soc Open Sci. 2016 Apr 20;3(4):160138. doi: 10.1098/rsos.160138. eCollection 2016 Apr. R Soc Open Sci. 2016. PMID: 27152227 Free PMC article. - Anthrax toxin component, Protective Antigen, protects insects from bacterial infections.
Alameh S, Bartolo G, O'Brien S, Henderson EA, Gonzalez LO, Hartmann S, Klimko CP, Shoe JL, Cote CK, Grill LK, Levitin A, Martchenko Shilman M. Alameh S, et al. PLoS Pathog. 2020 Aug 31;16(8):e1008836. doi: 10.1371/journal.ppat.1008836. eCollection 2020 Aug. PLoS Pathog. 2020. PMID: 32866212 Free PMC article. - Mosquito C-type lectins maintain gut microbiome homeostasis.
Pang X, Xiao X, Liu Y, Zhang R, Liu J, Liu Q, Wang P, Cheng G. Pang X, et al. Nat Microbiol. 2016 May;1:16023. doi: 10.1038/nmicrobiol.2016.23. Epub 2016 Mar 14. Nat Microbiol. 2016. PMID: 27170846 Free PMC article. - Peptidoglycan recognition proteins in Drosophila immunity.
Kurata S. Kurata S. Dev Comp Immunol. 2014 Jan;42(1):36-41. doi: 10.1016/j.dci.2013.06.006. Epub 2013 Jun 22. Dev Comp Immunol. 2014. PMID: 23796791 Free PMC article. Review.
References
- Hultmark D. Trends Genet. 1993;9:178–183. - PubMed
- Hoffmann J A. Curr Opin Immunol. 1995;7:4–10. - PubMed
- Tryselius Y, Samakovlis C, Kimbrell D A, Hultmark D. Eur J Biochem. 1992;204:395–399. - PubMed
- Wicker C, Reichhart J M, Hoffmann D, Hultmark D, Samakovlis C, Hoffmann J A. J Biol Chem. 1990;265:22493–22498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases