Two distinct pathways for targeting proteins from the cytoplasm to the vacuole/lysosome - PubMed (original) (raw)
Two distinct pathways for targeting proteins from the cytoplasm to the vacuole/lysosome
M Baba et al. J Cell Biol. 1997.
Abstract
Stress conditions lead to a variety of physiological responses at the cellular level. Autophagy is an essential process used by animal, plant, and fungal cells that allows for both recycling of macromolecular constituents under conditions of nutrient limitation and remodeling the intracellular structure for cell differentiation. To elucidate the molecular basis of autophagic protein transport to the vacuole/lysosome, we have undertaken a morphological and biochemical analysis of this pathway in yeast. Using the vacuolar hydrolase aminopeptidase I (API) as a marker, we provide evidence that the autophagic pathway overlaps with the biosynthetic pathway, cytoplasm to vacuole targeting (Cvt), used for API import. Before targeting, the precursor form of API is localized mostly in restricted regions of the cytosol as a complex with spherical particles (termed Cvt complex). During vegetative growth, the Cvt complex is selectively wrapped by a membrane sac forming a double membrane-bound structure of approximately 150 nm diam, which then fuses with the vacuolar membrane. This process is topologically the same as macroautophagy induced under starvation conditions in yeast (Baba, M., K. Takeshige, N. Baba, and Y. Ohsumi. 1994. J. Cell Biol. 124:903-913). However, in contrast with autophagy, API import proceeds constitutively in growing conditions. This is the first demonstration of the use of an autophagy-like mechanism for biosynthetic delivery of a vacuolar hydrolase. Another important finding is that when cells are subjected to starvation conditions, the Cvt complex is now taken up by an autophagosome that is much larger and contains other cytosolic components; depending on environmental conditions, the cell uses an alternate pathway to sequester the Cvt complex and selectively deliver API to the vacuole. Together these results indicate that two related but distinct autophagy-like processes are involved in both biogenesis of vacuolar resident proteins and sequestration of substrates to be degraded.
Figures
Figure 1
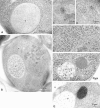
Morphological studies of proAPI in the cytosol. Cells were grown in YEPD medium at 30°C to log phase and were prepared for electron microscopy as described in Materials and Methods. Where indicated, immunostaining of API was performed using antibody to the mature protein that detects both the mature and precursor forms of the hydrolase. (A) Immunostaining of API in the vacuole of SEY6210 (wild-type) cell showing a dispersed pattern. (B–D) Immunostaining of TVY1 (pep4) cells showing API in the Cvt complex. The arrow points to a Cvt complex. Higher magnification images are shown in C and D. (E) Freeze-substitution fixation image of Cvt complex in TVY1 cell. (F) Freeze-substitution fixation image of a Cvt complex in strain SEY6210 transformed with a 2μ plasmid encoding API grown in SD medium. (G) Immunostaining of strain SEY6210 containing a 2μ APE1 plasmid grown in SD medium. N, Nucleus; V, vacuole.
Figure 2
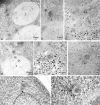
Membrane-bound forms of Cvt complex in TVY1 (pep4) cells. Cells were grown in YEPD medium at 30°C to log phase and prepared for electron microscopy as described in Fig. 1. (A and B) Immunostaining image showing Cvt vesicles (marked with arrows in A) in the cytosol. Arrowhead and double arrowheads show the inner and outer membrane of Cvt vesicle, respectively. (C) Freeze-substitution fixation image of Cvt vesicles (marked with arrows). (D) Immunostaining of Cvt vesicle (marked with arrows) in the cytosol and Cvt body (marked with double arrows) in the vacuole. (E and F) Freeze-substitution fixation image of Cvt bodies in the vacuole (marked with arrows). A Cvt vesicle is marked with an arrowhead. (G and H) Freeze-substitution fixation image of Cvt vesicle contacting and fusing to a vacuole. V, Vacuole; VM, vacuolar membrane.
Figure 7
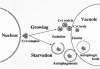
Scheme of API transport pathways to the vacuole. As described in the text, proAPI forms a complex in the cytosol. This Cvt complex is then targeted to the vacuole by two pathways depending upon the nutrient conditions. In growing conditions, a double membrane vesicle of ∼140 to 160 nm forms around the Cvt complex and excludes cytosol. In contrast, when cells are starved for nitrogen, the Cvt complex is taken up into larger autophagosomes (300–900 nm) together with cytosolic components. Vacuolar delivery is achieved by fusion of the outer membrane of the Cvt vesicle or autophagosome with the vacuolar membrane. Finally, the resulting Cvt bodies or autophagic bodies are broken down by vacuolar hydrolases to release proAPI into the vacuole lumen. The precursor form of the protein is then processed into the mature hydrolase.
Figure 4
Transport of Cvt complex in SEY6210 (wild-type) harboring 2μ plasmid encoding API under growing and nitrogen starvation conditions. Vegetative cells grown in SD medium were observed (A–E). For starvation, logarithmically growing cells in SD were transferred to SD(-N) for 25 min (F–H) or 1 h (I–K) in the presence of 1 mM PMSF. Samples were prepared for electron microscopy as described in Fig. 1. (A) Freeze-substitution fixation image of Cvt vesicle (marked with an arrow). (B) Immunostaining of a Cvt vesicle (marked with an arrow). (C and D) Higher magnification image of Cvt vesicle. Arrowhead and double arrowheads show the inner and outer membrane of a Cvt vesicle, respectively. (E) Freeze-substitution fixation image of a membrane sac enclosing small portion of a large Cvt complex (marked with an arrow). (F) Freeze-substitution image of the wrapping of a Cvt complex by the isolation membrane (marked with an arrow). (G) Freeze-substitution image of a Cvt vesicle (marked with an arrow) fusing to a vacuole. (H) Freeze-substitution image of autophagic body containing a Cvt complex. (I) Immunostaining image depicting the wrapping of a Cvt complex. The arrow marks the enwrapping membrane. (J) Immunostaining of an autophagosome containing a Cvt complex. (K) Immunostaining of an autophagic body containing a Cvt complex in the vacuole. V, vacuole.
Figure 3
Cvt complexes in TVY1 (pep4) cells under nitrogen starvation conditions. Logarithmically growing cells in YEPD were transferred to nitrogen starvation medium (SD[-N]) at 30°C for 30 min (A and B) or 60 min (C and D) and prepared for electron microscopy as described in Fig. 1. (A) Freeze-substitution fixation image showing a Cvt complex in an autophagosome (marked by an arrow) in the cytosol. (B) Freeze-substitution fixation image showing a Cvt complex in an autophagic body in the vacuole. (C) Immunostaining of a Cvt complex in an autophagic body in the vacuole. (D) Immunostaining of Cvt vesicles (marked with arrows) in the vacuole. AB, Autophagic body; AP, autophagosome; V, vacuole.
Figure 5
Mutant analysis allows for differentiation between vacuolar delivery by Cvt vesicles and autophagosomes. (A) SEY6210 (wild-type), cvt3, and cvt7 yeast were grown in SD to 1 OD600 U/ml and transferred to SD (-N). Aliquots were collected after 0 h (+) and 4 h (−) incubation and subjected to immunoblotting. The positions of precursor (pro) and mature (m) API are indicated. (B) cvt3 and cvt7 cells were pulse labeled for 10 min. After addition of cold cysteine and methionine, the cells were harvested, washed, and resuspended in either SD or SD(-N) and chased for the indicated times. API was recovered by immunoprecipitation, resolved on SDS polyacrylamide gels, and quantified using a phosphorimager (STORM; Molecular Dynamics, Sunnyvale, CA).
Figure 5
Mutant analysis allows for differentiation between vacuolar delivery by Cvt vesicles and autophagosomes. (A) SEY6210 (wild-type), cvt3, and cvt7 yeast were grown in SD to 1 OD600 U/ml and transferred to SD (-N). Aliquots were collected after 0 h (+) and 4 h (−) incubation and subjected to immunoblotting. The positions of precursor (pro) and mature (m) API are indicated. (B) cvt3 and cvt7 cells were pulse labeled for 10 min. After addition of cold cysteine and methionine, the cells were harvested, washed, and resuspended in either SD or SD(-N) and chased for the indicated times. API was recovered by immunoprecipitation, resolved on SDS polyacrylamide gels, and quantified using a phosphorimager (STORM; Molecular Dynamics, Sunnyvale, CA).
Figure 6
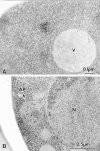
Analysis of the Cvt complex in the cvt3 mutant under vegetative and starvation conditions. Vegetative cells were grown in SD medium at 30°C to log phase. For starvation, logarithmically growing cells in SD medium were transferred to SD(-N) for 5 h. Samples were prepared for electron microscopy as described in Fig. 1. (A) Immunostaining image depicting Cvt complex in the cytosol. Cvt vesicles are not detected in this strain. (B) Freeze-substitution fixation image showing Cvt complex in autophagosome (marked with an arrow) under starvation conditions, indicating that autophagy can still proceed. AP, Autophagosome; N, nucleus; V, vacuole.
Similar articles
- Transport of proteins to the yeast vacuole: autophagy, cytoplasm-to-vacuole targeting, and role of the vacuole in degradation.
Teter SA, Klionsky DJ. Teter SA, et al. Semin Cell Dev Biol. 2000 Jun;11(3):173-9. doi: 10.1006/scdb.2000.0163. Semin Cell Dev Biol. 2000. PMID: 10906274 Review. - Cytoplasm to vacuole trafficking of aminopeptidase I requires a t-SNARE-Sec1p complex composed of Tlg2p and Vps45p.
Abeliovich H, Darsow T, Emr SD. Abeliovich H, et al. EMBO J. 1999 Nov 1;18(21):6005-16. doi: 10.1093/emboj/18.21.6005. EMBO J. 1999. PMID: 10545112 Free PMC article. - Transport of a large oligomeric protein by the cytoplasm to vacuole protein targeting pathway.
Kim J, Scott SV, Oda MN, Klionsky DJ. Kim J, et al. J Cell Biol. 1997 May 5;137(3):609-18. doi: 10.1083/jcb.137.3.609. J Cell Biol. 1997. PMID: 9151668 Free PMC article. - Cytoplasm-to-vacuole targeting and autophagy employ the same machinery to deliver proteins to the yeast vacuole.
Scott SV, Hefner-Gravink A, Morano KA, Noda T, Ohsumi Y, Klionsky DJ. Scott SV, et al. Proc Natl Acad Sci U S A. 1996 Oct 29;93(22):12304-8. doi: 10.1073/pnas.93.22.12304. Proc Natl Acad Sci U S A. 1996. PMID: 8901576 Free PMC article. - Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells.
Kim J, Klionsky DJ. Kim J, et al. Annu Rev Biochem. 2000;69:303-42. doi: 10.1146/annurev.biochem.69.1.303. Annu Rev Biochem. 2000. PMID: 10966461 Review.
Cited by
- Visualization of Atg3 during autophagosome formation in Saccharomyces cerevisiae.
Ngu M, Hirata E, Suzuki K. Ngu M, et al. J Biol Chem. 2015 Mar 27;290(13):8146-53. doi: 10.1074/jbc.M114.626952. Epub 2015 Feb 2. J Biol Chem. 2015. PMID: 25645919 Free PMC article. - Atg17 functions in cooperation with Atg1 and Atg13 in yeast autophagy.
Kabeya Y, Kamada Y, Baba M, Takikawa H, Sasaki M, Ohsumi Y. Kabeya Y, et al. Mol Biol Cell. 2005 May;16(5):2544-53. doi: 10.1091/mbc.e04-08-0669. Epub 2005 Mar 2. Mol Biol Cell. 2005. PMID: 15743910 Free PMC article. - Cooperative binding of the cytoplasm to vacuole targeting pathway proteins, Cvt13 and Cvt20, to phosphatidylinositol 3-phosphate at the pre-autophagosomal structure is required for selective autophagy.
Nice DC, Sato TK, Stromhaug PE, Emr SD, Klionsky DJ. Nice DC, et al. J Biol Chem. 2002 Aug 16;277(33):30198-207. doi: 10.1074/jbc.M204736200. Epub 2002 Jun 4. J Biol Chem. 2002. PMID: 12048214 Free PMC article. - Cell-free reconstitution of microautophagic vacuole invagination and vesicle formation.
Sattler T, Mayer A. Sattler T, et al. J Cell Biol. 2000 Oct 30;151(3):529-38. doi: 10.1083/jcb.151.3.529. J Cell Biol. 2000. PMID: 11062255 Free PMC article. - Autophagy: molecular machinery for self-eating.
Yorimitsu T, Klionsky DJ. Yorimitsu T, et al. Cell Death Differ. 2005 Nov;12 Suppl 2(Suppl 2):1542-52. doi: 10.1038/sj.cdd.4401765. Cell Death Differ. 2005. PMID: 16247502 Free PMC article. Review.
References
- Baba M, Osumi M. Transmission and scanning electron microscopic examination of intracellular organelles in Freeze-substituted Kloeckera and Saccharomyces cerevisiaeyeast cells. J Electron Microsc Techn. 1987;5:249–261.
- Baba M, Osumi M, Ohsumi Y. Analysis of the membrane structures involved in autophagy in yeast by freeze-replica method. Cell Struct Funct. 1995;20:465–472. - PubMed
- Chiang H-L, Schekman R. Regulated import and degradation of a cytosolic protein in the yeast vacuole. Nature. 1991;350:313–318. - PubMed
- Chiang H-L, Schekman R, Hamamoto S. Selective uptake of cytosolic, peroxisomal, and plasma membrane proteins into the yeast lysosome for degradation. J Biol Chem. 1996;271:9934–9941. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases