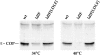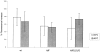Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component epsilon-COP - PubMed (original) (raw)
Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component epsilon-COP
E Daro et al. J Cell Biol. 1997.
Abstract
Recent evidence has suggested that subunits of the coatomer protein (COPI) complexes are functionally associated with endosomes in mammalian cells. We now provide genetic evidence that COPI plays a role in endocytosis in intact cells. The ldlF mutant CHO cell line bears a temperature-sensitive defect in the COPI subunit epsilon-COP. In addition to exhibiting conditional defects in the secretory pathway, we find that the cells are also defective at mediating endosome-associated functions. As found for cells microinjected with anti-COPI antibodies, ldlF cells at the restrictive temperature could not be infected by vesicular stomatitis (VSV) or Semliki Forest virus (SFV) that require delivery to acidic endosomes to penetrate into the cytosol. Although there was no temperature-sensitive defect in the internalization of receptor-bound transferrin (Tfn), Tfn recycling and accumulation of HRP were markedly inhibited at the restrictive temperature. Sorting of receptor-bound markers such as EGF to lysosomes was also reduced, although delivery of fluid-phase markers was only partially inhibited. In addition, lysosomes redistributed from their typical perinuclear location to the tips of the ldlF cells. Mutant phenotypes began to emerge within 2 h of temperature shift, the time required for the loss of detectable epsilon-COP, suggesting that the endocytic defects were not secondary to a block in the secretory pathway. Importantly, the mutant phenotypes were also corrected by transfection of wild-type epsilon-COP cDNA demonstrating that they directly or indirectly reflected the epsilon-COP defect. Taken together, the results suggest that epsilon-COP acts early in the endocytic pathway, most likely inhibiting the normal sorting and recycling functions of early endosomes.
Figures
Figure 1
ldlF cells lack ε-COP. After incubation of wt and mutant cells at either 34° or 40°C for 12 h, cell lysates were normalized for total cell protein and then subjected to SDS-PAGE and immunoblotting. At 34°C, ldlF cells have much less ε-COP than wt cells, and after incubation at 40°C for 12 h, ε-COP is undetectable in the mutant cells. The defect was complemented by transfection with wt ε-COP in ldlF[LDLF] cells.
Figure 2
Virus infection is inhibited in ldlF cells. After incubation for 10 h at 34° or 40°C, cells were exposed to SFV for 2.5 h to allow for viral endocytosis as described in Materials and Methods. Infection was monitored by immunofluorescence detection of newly synthesized SFV–E2 spike glycoprotein. SFV infection occurred normally in ldlF cells preincubated at 34°C and wt and ldlF[LDLF] cells preincubated at either temperature. wt and ldlF[LDLF] are representative of results obtained at either temperature. No viral infection was observed in ldlF cells that were preincubated at 40°C. Similar results were obtained for tsO45 mutant of VSV.
Figure 3
SFV does not reach an acidic compartment in ldlF cells. After SFV uptake for 30 min, delivery of SFV to an acidic compartment was monitored by immunofluorescence detection of the acid-specific (pH < 6.2) conformation of SFV–E1 protien. Cells were preincubated at 34° or 40°C for 10 h. The acid conformation was detected in ldlF cells preincubated at 34°C, wt, and ldlF[LDLF] cells, but not ldlF cells preincubated at 40°C. wt and ldlF[LDLF] are representative of results obtained at either temperature.
Figure 4
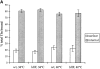
ldlF cells have a partial defect in transferrin endocytosis. (A) Loss of ε-COP does not affect the distribution of Tfn receptors. After incubation of wt and mutant cells at 34° and 40°C for 8 h, intracellular and cell surface receptors were saturated with [125I]Tfn by labeling for 60 min at 34° or 40°C. Surface and internal Tfn were determined as described in Materials and Methods. Values are represented as percent total of bound [125I]Tfn. In all conditions, cells exhibited a TfnR distribution with ∼20% on the cell surface, and 80% intracellular. n = 9. (B) Tfn internalization is inhibited in ldlF cells. After incubation of wt and mutant cells at 40°C for 8 h, cells were incubated with [125I]Tfn at 40°C for various amounts of time to allow for internalization of receptor-bound Tfn. Internalized Tfn was assayed after removal of cell surface Tfn (% surface-bound Tfn was similar for wt and ldlF cells) and normalized for total cell protein as described in Materials and Methods. Internalization of Tfn was inhibited in ldlF cells lacking ε-COP. n = 6. (C) Tfn internalization defect is complemented in ldlF[LDLF] cells and is partially temperature sensitive. After incubation at 34° or 40°C for 8 h, cells were allowed to internalize [125I]Tfn for 5 min and internalized Tfn was assayed as in B. wt cells internalized similar amounts of [125I]Tfn at either temperature, but ldlF cells internalized somewhat less [125I]Tfn at 34°C and even less at 40°C as compared to wt cells. The defect was complemented by addition of wt ε-COP in ldlF[LDLF] cells. n = 6.
Figure 4
ldlF cells have a partial defect in transferrin endocytosis. (A) Loss of ε-COP does not affect the distribution of Tfn receptors. After incubation of wt and mutant cells at 34° and 40°C for 8 h, intracellular and cell surface receptors were saturated with [125I]Tfn by labeling for 60 min at 34° or 40°C. Surface and internal Tfn were determined as described in Materials and Methods. Values are represented as percent total of bound [125I]Tfn. In all conditions, cells exhibited a TfnR distribution with ∼20% on the cell surface, and 80% intracellular. n = 9. (B) Tfn internalization is inhibited in ldlF cells. After incubation of wt and mutant cells at 40°C for 8 h, cells were incubated with [125I]Tfn at 40°C for various amounts of time to allow for internalization of receptor-bound Tfn. Internalized Tfn was assayed after removal of cell surface Tfn (% surface-bound Tfn was similar for wt and ldlF cells) and normalized for total cell protein as described in Materials and Methods. Internalization of Tfn was inhibited in ldlF cells lacking ε-COP. n = 6. (C) Tfn internalization defect is complemented in ldlF[LDLF] cells and is partially temperature sensitive. After incubation at 34° or 40°C for 8 h, cells were allowed to internalize [125I]Tfn for 5 min and internalized Tfn was assayed as in B. wt cells internalized similar amounts of [125I]Tfn at either temperature, but ldlF cells internalized somewhat less [125I]Tfn at 34°C and even less at 40°C as compared to wt cells. The defect was complemented by addition of wt ε-COP in ldlF[LDLF] cells. n = 6.
Figure 4
ldlF cells have a partial defect in transferrin endocytosis. (A) Loss of ε-COP does not affect the distribution of Tfn receptors. After incubation of wt and mutant cells at 34° and 40°C for 8 h, intracellular and cell surface receptors were saturated with [125I]Tfn by labeling for 60 min at 34° or 40°C. Surface and internal Tfn were determined as described in Materials and Methods. Values are represented as percent total of bound [125I]Tfn. In all conditions, cells exhibited a TfnR distribution with ∼20% on the cell surface, and 80% intracellular. n = 9. (B) Tfn internalization is inhibited in ldlF cells. After incubation of wt and mutant cells at 40°C for 8 h, cells were incubated with [125I]Tfn at 40°C for various amounts of time to allow for internalization of receptor-bound Tfn. Internalized Tfn was assayed after removal of cell surface Tfn (% surface-bound Tfn was similar for wt and ldlF cells) and normalized for total cell protein as described in Materials and Methods. Internalization of Tfn was inhibited in ldlF cells lacking ε-COP. n = 6. (C) Tfn internalization defect is complemented in ldlF[LDLF] cells and is partially temperature sensitive. After incubation at 34° or 40°C for 8 h, cells were allowed to internalize [125I]Tfn for 5 min and internalized Tfn was assayed as in B. wt cells internalized similar amounts of [125I]Tfn at either temperature, but ldlF cells internalized somewhat less [125I]Tfn at 34°C and even less at 40°C as compared to wt cells. The defect was complemented by addition of wt ε-COP in ldlF[LDLF] cells. n = 6.
Figure 5
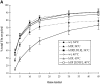
Transferrin recycling is defective in ldlF cells. (A) Loss of ε-COP inhibits Tfn recycling. After incubation at 34° and 40°C for ⩽12 h, cells were labeled to steady state with [125I]Tfn and then transferred to ice and washed extensively. Recycling was then initiated by warming the cells to 34° or 40°C and the medium was assayed at various time points. After 60 min, the remaining cell-associated [125I]Tfn was determined by lysing the cells in 1% Triton X-100. Total [125I]Tfn is the sum of cell associated and recycled Tfn at each time point. Values from each time point were normalized to % total Tfn. There is a slight slowing in the rate of Tfn recycling in ldlF cells compared to wt and ldlF[LDLF] cells at 34°C. There is a significant inhibition of recycling at 40°C in the ε-COP–deficient ldlF cells and the defect is complemented in ldlF[LDLF] cells at 40°C. n = 15 for wt and ldlF cells and n = 6 for ldlF[LDLF] cells. (B) The recycling defect occurs within 1 h of shift to 40°C. wt and ldlF cells were preincubated at 40°C for 0–8 h before measuring the amount of [125I]Tfn recycled at the 15 min time point in A. Recycling decreased within 1 h of shifting to the nonpermissive temperature, and the inhibition was qualitatively half-maximal by 2 h. n = 3.
Figure 5
Transferrin recycling is defective in ldlF cells. (A) Loss of ε-COP inhibits Tfn recycling. After incubation at 34° and 40°C for ⩽12 h, cells were labeled to steady state with [125I]Tfn and then transferred to ice and washed extensively. Recycling was then initiated by warming the cells to 34° or 40°C and the medium was assayed at various time points. After 60 min, the remaining cell-associated [125I]Tfn was determined by lysing the cells in 1% Triton X-100. Total [125I]Tfn is the sum of cell associated and recycled Tfn at each time point. Values from each time point were normalized to % total Tfn. There is a slight slowing in the rate of Tfn recycling in ldlF cells compared to wt and ldlF[LDLF] cells at 34°C. There is a significant inhibition of recycling at 40°C in the ε-COP–deficient ldlF cells and the defect is complemented in ldlF[LDLF] cells at 40°C. n = 15 for wt and ldlF cells and n = 6 for ldlF[LDLF] cells. (B) The recycling defect occurs within 1 h of shift to 40°C. wt and ldlF cells were preincubated at 40°C for 0–8 h before measuring the amount of [125I]Tfn recycled at the 15 min time point in A. Recycling decreased within 1 h of shifting to the nonpermissive temperature, and the inhibition was qualitatively half-maximal by 2 h. n = 3.
Figure 6
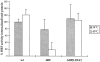
Endosomal pH is similar in wt, ldlF, and ldlF[LDLF] cells. After incubation of wt and mutant cells at 34° and 40°C for 8 h, cells were loaded with FITC–Tfn. FITC fluorescence was quantitated both before and after equilibration to pH 7.4 with nigericin. The recorded change in fluoresence intensity is proportional to the difference between the pH of the FITC–Tfn-containing endosomes and that of the equilibration buffer (pH 7.4). Within the limits of these experiments, there was no significant difference observed between ldlf mutant cells and either the wt or ldlf[LDLF] mutant cells. n = 8 for wt cells at 40°C, n = 9 for ldlF cells at 40°C, and n = 6 for all others.
Figure 7
Loss of ε-COP inhibits accumulation of fluid-phase markers. Cells were incubated for 8.5 h at either 34° or 40°C before labeling with the fluid-phase marker, HRP, for 90 min at either temperature. After extensive washing, cells were lysed and assayed for HRP activity and total cell protein. Loss of ε-COP in ldlF cells at 40°C resulted in an inhibition of HRP accumulation. The defect was restored in ldlF[LDLF] cells which were complemented with wt ε-COP. n = 12 for wt and ldlF cells and n = 6 for ldlF[LDLF] cells.
Figure 8
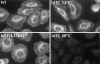
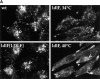
Lysosome, but not endosome, distribution is altered in ldlF cells. Cells were incubated at either 34° or 40°C to compare the distribution of internalized FITC–Tfn and the lysosomal glycoprotein lgp-B. (A) Distribution of lysosomes. ldlf and wt cells were fixed and immunostained for lgp-B. Wt cells exhibited the characteristic clustering of lysosomes in the perinuclear region (large arrowheads), whereas the lysosomes in the mutant cells seem to be more randomly dispersed at the permissive temperature. Upon incubation at the nonpermissive temperature for ⩽12 h, the lysosomes of the mutant cells seem to redistribute to the cell periphery (small arrowheads), extending to the very tips of the cells. Occasionally, mutant cells were observed to display both centrally (large arrowheads, bottom right) and peripherally (small arrowheads, bottom right) clustered lysosomes. Addition of wt ε-COP in the ldlF[LDLF] cells restored this phenotype (large arrowheads). Similar results were obtained when cells were incubated at 40°C for only 5 h. wt and ldlF[LDLF] panels are representative of results obtained at either temperature. (B) Distribution of FITC–Tfn-containing early endosomes. Wt, ldlf, and ldlf[LDLF] cells were incubated at the permissive or nonpermissive temperature for 12 h and then allowed to internalize FITC– Tfn for 1 h. Most (>80%) wild-type and 30–60% of ldlf CHO cells exhibited the characteristic clustering of recycling endosomes at the pericentriolar region (arrows) at both temperatures. ldlF[LDLF] cells were similar to wt cells. Arrows indicate the position of the PNRC. There was no redistribution of Tfn-containing endosomes to the cell periphery.
Figure 8
Lysosome, but not endosome, distribution is altered in ldlF cells. Cells were incubated at either 34° or 40°C to compare the distribution of internalized FITC–Tfn and the lysosomal glycoprotein lgp-B. (A) Distribution of lysosomes. ldlf and wt cells were fixed and immunostained for lgp-B. Wt cells exhibited the characteristic clustering of lysosomes in the perinuclear region (large arrowheads), whereas the lysosomes in the mutant cells seem to be more randomly dispersed at the permissive temperature. Upon incubation at the nonpermissive temperature for ⩽12 h, the lysosomes of the mutant cells seem to redistribute to the cell periphery (small arrowheads), extending to the very tips of the cells. Occasionally, mutant cells were observed to display both centrally (large arrowheads, bottom right) and peripherally (small arrowheads, bottom right) clustered lysosomes. Addition of wt ε-COP in the ldlF[LDLF] cells restored this phenotype (large arrowheads). Similar results were obtained when cells were incubated at 40°C for only 5 h. wt and ldlF[LDLF] panels are representative of results obtained at either temperature. (B) Distribution of FITC–Tfn-containing early endosomes. Wt, ldlf, and ldlf[LDLF] cells were incubated at the permissive or nonpermissive temperature for 12 h and then allowed to internalize FITC– Tfn for 1 h. Most (>80%) wild-type and 30–60% of ldlf CHO cells exhibited the characteristic clustering of recycling endosomes at the pericentriolar region (arrows) at both temperatures. ldlF[LDLF] cells were similar to wt cells. Arrows indicate the position of the PNRC. There was no redistribution of Tfn-containing endosomes to the cell periphery.
Figure 9
Peripheral lysosomes are accessible to fluid-phase markers in ldlF cells. To determine if the peripheral lysosomes in Fig. 7 were accessible to a fluid-phase tracer, cells were first incubated for 6 h at 40°C and then labeled with LY for 90 min and washed for 90 min at 40°C. The cells were then fixed and stained for lysosomes using the lgp-B antibody shown in Fig. 7. In ldlF cells, the lysosomes (red) are found at the tips of the cells (arrowheads), but these structures are still accessible to internalized LY (green) as indicated by the yellow color in the merge panel.
Figure 10
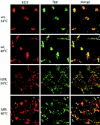
EGF does not reach lysosomes in ldlF cells. To follow the intracellular destination of a lysosomally targeted ligand, Texas red–conjugated EGF was internalized for 30 min after the cells were first preincubated for ⩽12 h at 34° or 40°C. The cells were then fixed and counter-stained for lysosomes using the same lgp-B antibody shown in Figs. 7 and 8. In wt cells at either temperature, the internalized EGF (red) reached lgp-positive structures (green). Overlap is yellow. In ldlF cells, there is very little overlap between the red and green channels at either temperature, indicating that very little of the internalized EGF reached lgp-positive structures. In ldlF cells at 40°C, the lysosomes (green) are found at the tips of the cells (arrowheads), but EGF (red) is not found in these structures.
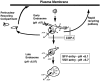
Figure 11
Potential sites for COP-I affected steps in endosomal transport. Arrows indicate pathways of endosomal traffic. Double lines indicate pathways that may be blocked when COP-I is inactivated. Recycling of receptors may occur through both a rapid return pathway from the early endosomes and a slower pathway passing through the PNRC. COP-I may be involved in budding and/or sorting events in the early endosome required for normal trafficking to late endosomes and through the rapid return pathway.
Similar articles
- Induction of direct endosome to endoplasmic reticulum transport in Chinese hamster ovary (CHO) cells (LdlF) with a temperature-sensitive defect in epsilon-coatomer protein (epsilon-COP).
Llorente A, Lauvrak SU, van Deurs B, Sandvig K. Llorente A, et al. J Biol Chem. 2003 Sep 12;278(37):35850-5. doi: 10.1074/jbc.M303425200. Epub 2003 Jul 7. J Biol Chem. 2003. PMID: 12847103 - Acidification of endosome subpopulations in wild-type Chinese hamster ovary cells and temperature-sensitive acidification-defective mutants.
Schmid S, Fuchs R, Kielian M, Helenius A, Mellman I. Schmid S, et al. J Cell Biol. 1989 Apr;108(4):1291-300. doi: 10.1083/jcb.108.4.1291. J Cell Biol. 1989. PMID: 2925786 Free PMC article. - A single point mutation in epsilon-COP results in temperature-sensitive, lethal defects in membrane transport in a Chinese hamster ovary cell mutant.
Guo Q, Penman M, Trigatti BL, Krieger M. Guo Q, et al. J Biol Chem. 1996 May 10;271(19):11191-6. doi: 10.1074/jbc.271.19.11191. J Biol Chem. 1996. PMID: 8626666 - Investigation of endosome and lysosome biology by ultra pH-sensitive nanoprobes.
Wang C, Zhao T, Li Y, Huang G, White MA, Gao J. Wang C, et al. Adv Drug Deliv Rev. 2017 Apr;113:87-96. doi: 10.1016/j.addr.2016.08.014. Epub 2016 Sep 6. Adv Drug Deliv Rev. 2017. PMID: 27612550 Free PMC article. Review. - Strategy for Cytoplasmic Delivery Using Inorganic Particles.
Xu ZPG. Xu ZPG. Pharm Res. 2022 Jun;39(6):1035-1045. doi: 10.1007/s11095-022-03178-1. Epub 2022 Feb 2. Pharm Res. 2022. PMID: 35112228 Free PMC article. Review.
Cited by
- The TOR complex 1 is distributed in endosomes and in retrograde vesicles that form from the vacuole membrane and plays an important role in the vacuole import and degradation pathway.
Brown CR, Hung GC, Dunton D, Chiang HL. Brown CR, et al. J Biol Chem. 2010 Jul 23;285(30):23359-70. doi: 10.1074/jbc.M109.075143. Epub 2010 May 10. J Biol Chem. 2010. PMID: 20457600 Free PMC article. - Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170.
Valetti C, Wetzel DM, Schrader M, Hasbani MJ, Gill SR, Kreis TE, Schroer TA. Valetti C, et al. Mol Biol Cell. 1999 Dec;10(12):4107-20. doi: 10.1091/mbc.10.12.4107. Mol Biol Cell. 1999. PMID: 10588646 Free PMC article. - Role of the Guanine Nucleotide Exchange Factor GBF1 in the Replication of RNA Viruses.
Martínez JL, Arias CF. Martínez JL, et al. Viruses. 2020 Jun 24;12(6):682. doi: 10.3390/v12060682. Viruses. 2020. PMID: 32599855 Free PMC article. Review. - Involvement of specific COPI subunits in protein sorting from the late endosome to the vacuole in yeast.
Gabriely G, Kama R, Gerst JE. Gabriely G, et al. Mol Cell Biol. 2007 Jan;27(2):526-40. doi: 10.1128/MCB.00577-06. Epub 2006 Nov 13. Mol Cell Biol. 2007. PMID: 17101773 Free PMC article. - AGAP2 regulates retrograde transport between early endosomes and the TGN.
Shiba Y, Römer W, Mardones GA, Burgos PV, Lamaze C, Johannes L. Shiba Y, et al. J Cell Sci. 2010 Jul 15;123(Pt 14):2381-90. doi: 10.1242/jcs.057778. Epub 2010 Jun 15. J Cell Sci. 2010. PMID: 20551179 Free PMC article.
References
- Apodaca G, Aroeti B, Tang K, Mostov KE. Brefeldin-A inhibits the delivery of the polymeric immunoglobulin receptor to the basolateral surface of MDCK cells. J Biol Chem. 1993;268:20380–20385. - PubMed
- Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1632. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials