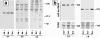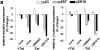Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells - PubMed (original) (raw)
Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells
A Lochter et al. J Cell Biol. 1997.
Abstract
Matrix metalloproteinases (MMPs) regulate ductal morphogenesis, apoptosis, and neoplastic progression in mammary epithelial cells. To elucidate the direct effects of MMPs on mammary epithelium, we generated functionally normal cells expressing an inducible autoactivating stromelysin-1 (SL-1) transgene. Induction of SL-1 expression resulted in cleavage of E-cadherin, and triggered progressive phenotypic conversion characterized by disappearance of E-cadherin and catenins from cell-cell contacts, downregulation of cytokeratins, upregulation of vimentin, induction of keratinocyte growth factor expression and activation, and upregulation of endogenous MMPs. Cells expressing SL-1 were unable to undergo lactogenic differentiation and became invasive. Once initiated, this phenotypic conversion was essentially stable, and progressed even in the absence of continued SL-1 expression. These observations demonstrate that inappropriate expression of SL-1 initiates a cascade of events that may represent a coordinated program leading to loss of the differentiated epithelial phenotype and gain of some characteristics of tumor cells. Our data provide novel insights into how MMPs function in development and neoplastic conversion.
Figures
Figure 7
Activation and induction of endogenous MMPs by SL-1. Negative images of gelatin zymograms of medium conditioned by SCp2, p2C, p2S7, and p2S10 cells maintained for 3 d in the presence (+) or absence (−) of Tet. Positions of molecular mass markers are indicated.
Figure 1
Effects of SL-1 on cell scattering, lactogenic differentiation, and invasion of mammary epithelial cells. (a) P2C, p2S7, and p2S10 cells were maintained for 3 d in the presence (+) or absence (−) of Tet. Medium conditioned by cells for 2 d was analyzed for expression of the SL-1 transgene by immunoblotting with a monoclonal antibody against the HA.11 epitope tag. Both latent SL-1 proenzyme (61 kD, top arrowhead; also in b–d) and active SL-1 (49 kD, bottom arrowhead; also in b–d) could be detected in conditioned medium from p2S7 and p2S10 cells, but not p2C cells. (b) Medium conditioned by p2S10 cells for 1 d was analyzed for the presence of SL-1 protein 1, 2, 3, and 4 d after omission of Tet from the culture medium (days of ind.) by immunoblotting with antibodies against the HA.11 epitope tag. (c) p2S10 cells were maintained for 3 d in the presence of increasing concentrations of Tet. Medium conditioned by cells for 2 d was analyzed for expression of the SL-1 transgene by immunoblotting with antibodies against the HA.11 epitope tag: 300 nM Tet was sufficient to completely inhibit SL-1 induction in p2S10 cells. (d) p2S10 cells were maintained for 3 d in the presence (+) or absence (−) of Tet and in the presence (+) or absence (−) of GM6001 (GM). Medium conditioned by cells for 2 d was analyzed for expression and activation of the SL-1 transgene by casein substrate gel zymography. Zymograms are shown as negative images. Note that activation, but not production of SL-1 is inhibited by GM6001. (e) Immunoblot detection of β-casein in SCp2, p2C, p2S7, and p2S10 cells cultured for 6 d with Matrigel and lactogenic hormones and in the presence (+) or absence (−) of Tet. (f) P2C, p2S7, and p2S10 cells were maintained for 12 d in the presence (+ Tet) or absence (− Tet) of Tet, fixed and stained with toluidine blue. (g) Invasion of Matrigel by SCp2, p2C, p2S7, and p2S10 cells in the presence (white bars) or absence (black bars) of Tet, or in the absence of Tet and in the presence of GM6001 (hatched bars; GM). Means and standard deviations from three independent experiments are shown. (h) Invasion of p2S7 (○) and p2S10 (▪) cells maintained in the absence of Tet for 2, 3, and 4 d after plating onto Matrigel. Means and standard deviations from three independent experiments are shown. Bar, 20 μM.
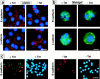
Figure 2
Subcellular localization of E-cadherin and β-catenin after SL-1 induction. (a–b) P2S10 cells were maintained for 6 d in the absence (a) or presence (b) of Matrigel and in the presence (+ Tet) or absence (− Tet) of Tet. Cells were analyzed by indirect immunofluorescence for distribution of E-cadherin and β-catenin (red in a, green in b). Cells were counterstained with DAPI to visualize nuclei (blue). (c) SCp2 cells were labeled with DiI (red) and cocultured with p2S10 cells for 6 d in the presence (+ Tet) or absence (− Tet) of Tet. Cells were then permeabilzed with digitonin, fixed, and then immunostained for E-cadherin (green). Cells were counterstained with DAPI (blue). Bars: (a and b) 10 μM; (c) 25 μm.
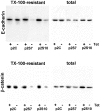
Figure 3
Total and cytoskeleton-associated E-cadherin and β-catenin after SL-1 induction. P2C, p2S7 and p2S10 cells were cultured for 6 d on tissue culture plastic in the presence (+) or absence (−) of Tet. They were then either directly lysed in RIPA buffer (total) or briefly extracted with Triton X-100 before cell lysis in RIPA buffer (TX-100-resistant) before analysis of expression of E-cadherin and β-catenin by immunoblotting.
Figure 4
Expression of E-cadherin and β-catenin in p2S cells cultured in the absence and presence of Matrigel. (a) Quantification of E-cadherin (○), β-catenin (▪), and γ-catenin (▴) expression by ELISA on p2S10 cells maintained on plastic and induced to express SL-1 for 2, 4, and 6 d (2d, 4d, and 6d) or 2 mo (2m), or on p2S10 cells induced to express SL-1 for 6 d in the presence of Matrigel. Results are expressed as percent increase or decrease in expression of cells maintained in the absence of Tet as compared to cells maintained in the presence of Tet. Means and standard deviations from three independent experiments are shown. (b) Quantification of β-catenin expression by ELISA on p2C (white bars), p2S7 (gray bars), and p2S10 (black bars) cells maintained on tissue culture plastic for 6 d in the presence (+ Tet) or in the absence (− Tet) of Tet with no further additives (none) or with GM6001 or its inactive homologue GM1210. Results are normalized with expression levels obtained in the presence of Tet set to 100. Means and standard deviations from three independent experiments are shown. (c) Immunoblots for total E-cadherin (E-cad) and β-catenin (β-cat) expressed by p2C and p2S10 cells maintained for 6 d (6d) or 2 mo (2m) in the presence (+) or absence (−) of Tet. (d) [35S]methionine-labeled SCp2 cells were treated for 12 h with conditioned medium from p2S10 cells induced (− Tet) or not induced (+ Tet) to express SL-1 for 6 d. The conditioned medium from p2S10 cells maintained without Tet was either supplemented (+ GM) or not supplemented (− GM) with GM6001. E-cadherin was subsequently immunoprecipitated from the cell culture medium with a monoclonal antibody against the extracellular (ECCD-2) or intracellular (C20820) domain of E-cadherin. The amount of cleaved E-cadherin detected in the medium, however, constituted only a minor part of the total E-cadherin produced by p2S cells (not shown). (e) Cell surface proteins of [35S]methionine-labeled p2S10 cells maintained for 6 d in the presence of Tet (6d +), or for 6 d (6d −), or 2 mo (2m −) in the absence of Tet were biotinylated, immunoprecipitated with antibodies against E-cadherin, and then visualized with peroxidase-conjugated streptavidin (cell surface). Autoradiograms of total immunoprecipitated E-cadherin protein (total), on the same membranes as used for immunoblotting, are shown for comparison.
Figure 5
Effect of SL-1 induction on expression of integrins. (a) Cell lysates of [35S]methionine-labeled p2S10 cells maintained for 6 d in the presence of Tet (6d +), or for 6 d (6d −), or 2 mo (2m −) in the absence of Tet were immunoprecipitated with antibodies against integrin subunits β1, β4, and α6. Positions of molecular mass markers of 220, 97.4, and 66 kD are indicated. (b) Cell surface proteins of [35S]methionine-labeled p2S10 cells maintained for 6 d in the presence of Tet (6d +), or for 6 d (6d −), or 2 mo (2m −) in the absence of Tet were biotinylated, immunoprecipitated with antibodies against integrin subunits β4 and α6, and visualized with peroxidase-conjugated streptavidin (cell surface). Autoradiograms of total immunoprecipitated β4 and α6 protein (total), on the same membranes as used for immunoblotting, are shown for comparison.
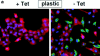
Figure 6
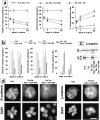
Effect of SL-1 induction on cytokeratin and vimentin expression. (a–b) P2S10 cells were maintained for 6 d in the absence (a) or presence (b) of Matrigel and in the presence (+ Tet) or absence (− Tet) of Tet. Cells were analyzed by indirect immunofluorescence for expression of cytokeratins (red) and vimentin (green). Cells were counterstained with DAPI (blue) to visualize nuclei. Note that in the presence of Matrigel most cells are round and form multicellular aggregates. (c) Quantification of cytokeratin expression by ELISA on p2C (•), p2S7 (▾), and p2S10 (▪) cells maintained on plastic and induced to express SL-1 for 2, 4, and 6 d (2d, 4d, and 6d), or 2 mo (2m), or on cells maintained in the presence of Matrigel for 6 d. Results are expressed as the percent increase or decrease in cytokeratin expression of cells maintained in the absence of Tet as compared to cells maintained in the presence of Tet. Means and standard deviations from three independent experiments are shown. (d) Quantification of the number of vimentin expressing p2C (circles), p2S7 (triangles) and p2S10 (squares) cells maintained on plastic or in the presence of Matrigel, and induced (black symbols) or not induced (white symbols) to express SL-1 for the times indicated above. Results are expressed as the percentage of cells displaying vimentin immunoreactivity. Means and standard deviations from three independent experiments are shown.
Figure 6
Effect of SL-1 induction on cytokeratin and vimentin expression. (a–b) P2S10 cells were maintained for 6 d in the absence (a) or presence (b) of Matrigel and in the presence (+ Tet) or absence (− Tet) of Tet. Cells were analyzed by indirect immunofluorescence for expression of cytokeratins (red) and vimentin (green). Cells were counterstained with DAPI (blue) to visualize nuclei. Note that in the presence of Matrigel most cells are round and form multicellular aggregates. (c) Quantification of cytokeratin expression by ELISA on p2C (•), p2S7 (▾), and p2S10 (▪) cells maintained on plastic and induced to express SL-1 for 2, 4, and 6 d (2d, 4d, and 6d), or 2 mo (2m), or on cells maintained in the presence of Matrigel for 6 d. Results are expressed as the percent increase or decrease in cytokeratin expression of cells maintained in the absence of Tet as compared to cells maintained in the presence of Tet. Means and standard deviations from three independent experiments are shown. (d) Quantification of the number of vimentin expressing p2C (circles), p2S7 (triangles) and p2S10 (squares) cells maintained on plastic or in the presence of Matrigel, and induced (black symbols) or not induced (white symbols) to express SL-1 for the times indicated above. Results are expressed as the percentage of cells displaying vimentin immunoreactivity. Means and standard deviations from three independent experiments are shown.
Figure 6
Effect of SL-1 induction on cytokeratin and vimentin expression. (a–b) P2S10 cells were maintained for 6 d in the absence (a) or presence (b) of Matrigel and in the presence (+ Tet) or absence (− Tet) of Tet. Cells were analyzed by indirect immunofluorescence for expression of cytokeratins (red) and vimentin (green). Cells were counterstained with DAPI (blue) to visualize nuclei. Note that in the presence of Matrigel most cells are round and form multicellular aggregates. (c) Quantification of cytokeratin expression by ELISA on p2C (•), p2S7 (▾), and p2S10 (▪) cells maintained on plastic and induced to express SL-1 for 2, 4, and 6 d (2d, 4d, and 6d), or 2 mo (2m), or on cells maintained in the presence of Matrigel for 6 d. Results are expressed as the percent increase or decrease in cytokeratin expression of cells maintained in the absence of Tet as compared to cells maintained in the presence of Tet. Means and standard deviations from three independent experiments are shown. (d) Quantification of the number of vimentin expressing p2C (circles), p2S7 (triangles) and p2S10 (squares) cells maintained on plastic or in the presence of Matrigel, and induced (black symbols) or not induced (white symbols) to express SL-1 for the times indicated above. Results are expressed as the percentage of cells displaying vimentin immunoreactivity. Means and standard deviations from three independent experiments are shown.
Figure 6
Effect of SL-1 induction on cytokeratin and vimentin expression. (a–b) P2S10 cells were maintained for 6 d in the absence (a) or presence (b) of Matrigel and in the presence (+ Tet) or absence (− Tet) of Tet. Cells were analyzed by indirect immunofluorescence for expression of cytokeratins (red) and vimentin (green). Cells were counterstained with DAPI (blue) to visualize nuclei. Note that in the presence of Matrigel most cells are round and form multicellular aggregates. (c) Quantification of cytokeratin expression by ELISA on p2C (•), p2S7 (▾), and p2S10 (▪) cells maintained on plastic and induced to express SL-1 for 2, 4, and 6 d (2d, 4d, and 6d), or 2 mo (2m), or on cells maintained in the presence of Matrigel for 6 d. Results are expressed as the percent increase or decrease in cytokeratin expression of cells maintained in the absence of Tet as compared to cells maintained in the presence of Tet. Means and standard deviations from three independent experiments are shown. (d) Quantification of the number of vimentin expressing p2C (circles), p2S7 (triangles) and p2S10 (squares) cells maintained on plastic or in the presence of Matrigel, and induced (black symbols) or not induced (white symbols) to express SL-1 for the times indicated above. Results are expressed as the percentage of cells displaying vimentin immunoreactivity. Means and standard deviations from three independent experiments are shown.
Figure 8
Induction of KGF by induction of SL-1. (a) Quantification of cytokeratin (keratin) and β-catenin expression by ELISA on SCp2 cells that had been incubated for 6 d with medium conditioned by p2C (white bars), p2S7 (gray bars), or p2S10 (black bars) cells maintained in the presence (+ Tet) or absence (− Tet) of Tet. GM6001 or GM1210 were added or not added (none) to conditioned medium from cells maintained in the absence of Tet. Results are expressed as the percent increase or decrease in cytokeratin or β-catenin expression compared to SCp2 cells maintained with conditioned medium from p2C cells cultured in the presence of Tet. Means and standard deviations from three independent experiments are shown. (b) Southern hybridization of RT-PCR products obtained with primers specific for HGF, KGF, or TIMP2 on total RNA prepared from p2C, p2S7, and p2S10 cells maintained for 3 days in the presence of Tet (3d +), or 3 d (3d −) or 2 months (2m −) in the absence of Tet, or from the mouse mammary carcinoma cell line SCg6 (g6).
Figure 8
Induction of KGF by induction of SL-1. (a) Quantification of cytokeratin (keratin) and β-catenin expression by ELISA on SCp2 cells that had been incubated for 6 d with medium conditioned by p2C (white bars), p2S7 (gray bars), or p2S10 (black bars) cells maintained in the presence (+ Tet) or absence (− Tet) of Tet. GM6001 or GM1210 were added or not added (none) to conditioned medium from cells maintained in the absence of Tet. Results are expressed as the percent increase or decrease in cytokeratin or β-catenin expression compared to SCp2 cells maintained with conditioned medium from p2C cells cultured in the presence of Tet. Means and standard deviations from three independent experiments are shown. (b) Southern hybridization of RT-PCR products obtained with primers specific for HGF, KGF, or TIMP2 on total RNA prepared from p2C, p2S7, and p2S10 cells maintained for 3 days in the presence of Tet (3d +), or 3 d (3d −) or 2 months (2m −) in the absence of Tet, or from the mouse mammary carcinoma cell line SCg6 (g6).
Figure 9
Irreversibility of effects of SL-1 on phenotypic conversion of p2S cells. (a) P2S10 cells were preinduced to express SL-1 for 6 d. Cells were then maintained on tissue culture plastic and in the presence or absence of Tet, or Tet and GM6001. Expression of β-catenin and cytokeratins (keratin) was determined by ELISA and the number of vimentin-positive cells was counted 1, 7, and 30 d later. ○, cells maintained in the presence of Tet all along; □, cells preinduced to express SL-1 for 6 d and subsequently maintained in the presence of Tet and GM6001; and ▾, cells preinduced to express SL-1 for 6 d and subsequently maintained in the absence of Tet. ELISA results were normalized with values obtained for cells maintained in the presence of Tet set to 100 at each time point. Means and standard deviations from three independent experiments are shown. (b) P2S10 cells were preinduced to express SL-1 for 6 d or 2 mo in the absence of Matrigel. Cells were then maintained with Matrigel and in the presence or absence of Tet or Tet and GM6001. Expression of β-catenin and cytokeratins (keratin) was determined by ELISA and the number of vimentin-positive cells was counted 1 and 7 d later. White bars, cells maintained in the presence of Tet all along; gray bars, cells not preinduced to express SL-1 and subsequently maintained in the absence of Tet; hatched bars, cells preinduced to express SL-1 for 6 d and subsequently maintained in the presence of Tet and GM6001; gray crosshatched bars, cells preinduced to express SL-1 for 2 mo and subsequently maintained in the presence of Tet and GM6001. ELISA results are normalized with values obtained for cells maintained in the presence of Tet set to 100 at each time point. Means and standard deviations from three independent experiments are shown. (c) Immunoblot analysis of β-casein expression by p2C and p2S10 cells maintained with Matrigel and lactogenic hormones for 7 d. Cells were either not preinduced to express SL-1, and then maintained with Matrigel in the presence (+ Tet) or absence (− Tet) of Tet, or were preinduced in the absence of Matrigel to express SL-1 for 6 d (6d − Tet, then + Tet + GM) or 2 mo (2m − Tet, then + Tet + GM), and then maintained in the presence of Tet and GM6001 with Matrigel. (d) Cells were either not preinduced to express SL-1 (+ Tet), or preinduced to express SL-1 for 6 d (− Tet 6d, then + Tet + GM) or 2 mo (− Tet 2m, then + Tet + GM) in the absence of Matrigel before addition of Tet and GM6001 together with Matrigel for 7 d. Cells were then analyzed by indirect immunofluorescence for distribution of E-cadherin and β-catenin. Bar, 10 μm.
Similar articles
- Cell-extracellular matrix interactions and EGF are important regulators of the basal mammary epithelial cell phenotype.
Deugnier MA, Faraldo MM, Rousselle P, Thiery JP, Glukhova MA. Deugnier MA, et al. J Cell Sci. 1999 Apr;112 ( Pt 7):1035-44. doi: 10.1242/jcs.112.7.1035. J Cell Sci. 1999. PMID: 10198285 - The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells.
Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ. Simian M, et al. Development. 2001 Aug;128(16):3117-31. doi: 10.1242/dev.128.16.3117. Development. 2001. PMID: 11688561 Free PMC article. - The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis.
Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. Sternlicht MD, et al. Cell. 1999 Jul 23;98(2):137-46. doi: 10.1016/s0092-8674(00)81009-0. Cell. 1999. PMID: 10428026 Free PMC article. - The significance of matrix metalloproteinases during early stages of tumor progression.
Lochter A, Sternlicht MD, Werb Z, Bissell MJ. Lochter A, et al. Ann N Y Acad Sci. 1998 Oct 23;857:180-93. doi: 10.1111/j.1749-6632.1998.tb10116.x. Ann N Y Acad Sci. 1998. PMID: 9917841 Review. - Matrix metalloproteinase-induced malignancy in mammary epithelial cells.
Stallings-Mann M, Radisky D. Stallings-Mann M, et al. Cells Tissues Organs. 2007;185(1-3):104-10. doi: 10.1159/000101310. Cells Tissues Organs. 2007. PMID: 17587815 Review.
Cited by
- Recent insights into natural product inhibitors of matrix metalloproteinases.
Kumar GB, Nair BG, Perry JJP, Martin DBC. Kumar GB, et al. Medchemcomm. 2019 Oct 7;10(12):2024-2037. doi: 10.1039/c9md00165d. eCollection 2019 Dec 1. Medchemcomm. 2019. PMID: 32904148 Free PMC article. Review. - The role of the tumor microenvironment in regulating angiogenesis.
Watnick RS. Watnick RS. Cold Spring Harb Perspect Med. 2012 Dec 1;2(12):a006676. doi: 10.1101/cshperspect.a006676. Cold Spring Harb Perspect Med. 2012. PMID: 23209177 Free PMC article. - CD10 Is Again Expressed at a Certain Stage during the Neoplastic Process of Bladder Transitional Cell Carcinomas.
Jang TJ. Jang TJ. Cancer Res Treat. 2012 Dec;44(4):262-6. doi: 10.4143/crt.2012.44.4.262. Epub 2012 Dec 31. Cancer Res Treat. 2012. PMID: 23341790 Free PMC article. - Immune Checkpoint Blockade Improves Chemotherapy in the PyMT Mammary Carcinoma Mouse Model.
Sirait-Fischer E, Olesch C, Fink AF, Berkefeld M, Huard A, Schmid T, Takeda K, Brüne B, Weigert A. Sirait-Fischer E, et al. Front Oncol. 2020 Sep 10;10:1771. doi: 10.3389/fonc.2020.01771. eCollection 2020. Front Oncol. 2020. PMID: 33014872 Free PMC article. - E-cadherin roles in animal biology: A perspective on thyroid hormone-influence.
Izaguirre MF, Casco VH. Izaguirre MF, et al. Cell Commun Signal. 2016 Nov 4;14(1):27. doi: 10.1186/s12964-016-0150-1. Cell Commun Signal. 2016. PMID: 27814736 Free PMC article. Review.
References
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development (Camb) 1993;117:1187–1198. - PubMed
- Basbaum CB, Werb Z. Focalized proteolysis: spatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr Opin Cell Biol. 1996;8:731–738. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous