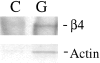The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures - PubMed (original) (raw)
The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures
I Rabinovitz et al. J Cell Biol. 1997.
Abstract
Functional studies on the alpha6beta4 integrin have focused primarily on its role in the organization of hemidesmosomes, stable adhesive structures that associate with the intermediate filament cytoskeleton. In this study, we examined the function of the alpha6beta4 integrin in clone A cells, a colon carcinoma cell line that expresses alpha6beta4 but no alpha6beta1 integrin and exhibits dynamic adhesion and motility on laminin-1. Time-lapse videomicroscopy of clone A cells on laminin-1 revealed that their migration is characterized by filopodial extension and stabilization followed by lamellae that extend in the direction of stabilized filopodia. A function-blocking mAb specific for the alpha6beta4 integrin inhibited clone A migration on laminin-1. This mAb also inhibited filopodial formation and stabilization and lamella formation. Indirect immunofluorescence microscopy revealed that the alpha6beta4 integrin is localized as discrete clusters in filopodia, lamellae, and retraction fibers. Although beta1 integrins were also localized in the same structures, a spatial separation of these two integrin populations was evident. In filopodia and lamellae, a striking colocalization of the alpha6beta4 integrin and F-actin was seen. An association between alpha6beta4 and F-actin is supported by the fact that alpha6beta4 integrin and actin were released from clone A cells by treatment with the F-actin- severing protein gelsolin and that alpha6beta4 immunostaining at the marginal edges of clone A cells on laminin-1 was resistant to solubilization with Triton X-100. Cytokeratins were not observed in filopodia and lamellipodia. Moreover, alpha6beta4 was extracted from these marginal edges with a Tween-40/deoxycholate buffer that solubilizes the actin cytoskeleton but not cytokeratins. Three other carcinoma cell lines (MIP-101, CCL-228, and MDA-MB-231) exhibited alpha6beta4 colocalized with actin in filopodia and lamellae. Formation of lamellae in these cells was inhibited with an alpha6-specific antibody. Together, these results indicate that the alpha6beta4 integrin functions in carcinoma migration on laminin-1 through its ability to promote the formation and stabilization of actin-containing motility structures.
Figures
Figure 1
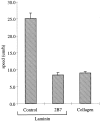
Random migration of clone A cells on laminin-1 is dependent on the integrin α6β4. Clone A cells were plated on laminin-1 or collagen I-coated dishes (10 μg/ ml) and incubated at 37°C for 30 min before the addition of either a control mouse IgG (10 μg/ml) or the α6 integrin–specific mAb 2B7 (10 μg/ml). Migration was analyzed by time-lapse videomicroscopy as described in the Materials and Methods section. The mean cell speed (i.e., displacement of the cell centroid as a function of time) obtained from the analysis of 30–40 cells for each experimental condition is reported in this bar graph. Error bars represent SEM.
Figure 2
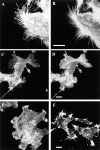
The motile morphology of clone A cells on laminin-1 is dependent on the integrin α6β4. Cells were plated on either laminin- 1–coated (A and C) or collagen I–coated (D) dishes and incubated at 37°C for 30 min before the addition of either (A) control mouse IgG (10 μg/ml) or (C) 2B7 mAb (10 μg/ml). After 30 min, the cells were photographed using phase contrast optics. Note the presence of prominent fan-shaped lamellae in cells on laminin-1 (A) and their digitally traced area in B. The α6-specific mAb 2B7 inhibits formation of these lamellae (C). Prominent lamellae are not seen when clone A cells are plated on collagen I (D). Bar, 20 μm.
Figure 3
Dynamics of filopodia and lamellae in clone A cells on laminin-1. Cells plated on laminin-1 were analyzed by time-lapse videomicroscopy. Each column shown (A–C) represents a sequence of frames recorded at the specified times from different cells migrating on laminin-1. Arrowheads indicate points at which the filopodia stabilize on the laminin-1 matrix. Asterisks denote the protruding lamella. In A, the filopodium shown stabilized at 2 min and formed an angle at the point indicated by the arrowhead. Note that only the anchoring point is attached because both proximal and distal segments shift positions at later times, during which the lamella extended following the direction of the filopodium. In B and C, the filopodia shown stabilized at 4 and 2 min, respectively. In contrast to the filopodium shown in A, the entire segment of these filopodia proximal to the vertex of the angle became immobilized and the lamellae extended in the direction of these stabilized filopodia. Bar, 5 μm.
Figure 4
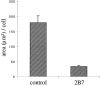
Formation of lamellae in clone A cells on laminin-1 requires the α6β4 integrin. Clone A cells were plated on laminin-1–coated dishes and incubated for 30 min at 37°C before the addition of 2B7 (10 μg/ml) or a control IgG. The cells were photographed after 1 h, and their lamellar area (μm2/cell) was determined by digital image analysis (see example in Fig. 2_B_). 50 cells were analyzed for each condition. Error bar represents SEM.
Figure 5
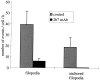
Inhibition of the integrin α6β4 reduces the formation and stabilization of filopodia in clone A cells on laminin-1. Clone A cells were plated on laminin-1–coated dishes and incubated for 30 min at 37°C before the addition of either 2B7 (10 μg/ml) or a control IgG (10 μg/ml) and analysis by time-lapse videomicroscopy. For each condition, five cells were monitored for 1 h at a frequency of one frame per minute and each frame was analyzed for the active formation of filopodia. A filopodium was considered stabilized if it remained immobile for several frames. The data shown represent the number of filopodia that either formed or stabilized/cell/hour. Error bar represents SEM.
Figure 6
The integrin α6β4 is localized in lamellae and filopodia in clone A cells on laminin-1. Cells plated on laminin-1 (A, B, and D) or collagen I (C) were fixed and processed for immunofluorescence using the rat GoH3 (anti-α6) mAb followed by a rhodamine-conjugated anti–rat antibody as described in the Materials and Methods section. The confocal images shown represent optical sections of the ventral surface. (A) Note the presence of α6β4 staining on the lamellae and in the filopodia at the leading edge (right side of the cell), as well as in retraction fibers at the trailing edge (left side of cell). (B) A higher magnification demonstrates the clustered appearance of α6β4 integrin in the filopodia and its presence at points of filopodial angling. (D) Fibers that are positive for α6β4 staining (arrowheads) are apparently left behind by the advancing cell on the left. (C) Clone A cells plated on collagen I exhibit only a diffuse pattern of α6β4 staining on their ventral surface. Bars, 10 μm.
Figure 7
Distinct localization of α6β4 and β1 integrins in the filopodia and lamellae of clone A cells on laminin-1. Clone A cells were plated on laminin-1 for 1 h at 37°C and processed for double immunofluorescence as described using rat GoH3 mAb and the mouse K-20 mAb followed by a combination of a TRITC-conjugated anti–rat antibody and an FITC-conjugated anti–mouse antibody that do not cross-react. The ventral surface of the cells was analyzed by confocal microscopy. Red, GoH3 mAb; green, K-20 mAb; yellow, colocalization. (A) Several filopodia and retraction fibers show a segregated distribution of α6β4 and β1 staining. (B) In lamellae, α6β4 staining is largely segregated from β1 staining except in the streak-shaped areas where filopodia project into the lamella. (C and D) Higher magnification images of filopodia showing the spatial segregation of α6β4 and β1 staining and the presence of α6β4 in the angles of filopodia (arrowheads). Bar, 5 μm.
Figure 8
Enhanced localization of the α6β4 integrin in retraction fibers of clone A cells on laminin-1. Cells plated on laminin-1 for 1 h at 37°C were processed for double immunofluorescence as described using the rat GoH3 mAb and mouse K-20 mAb followed by a combination of a TRITC-conjugated anti–rat antibody and an FITC-conjugated anti–mouse antibody that do not cross-react. The ventral surface of the cells was analyzed by confocal microscopy. (A) GoH3 mAb. (B) K-20 mAb. Note the presence of α6β4 staining in retraction fibers (A, arrowheads) at the trailing edge of the cells. Staining of β1 integrin is absent in these fibers (B). Bar, 10 μm.
Figure 9
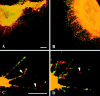
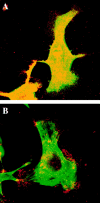
α6β4 integrin colocalizes with F-actin in filopodia of clone A cells on laminin-1. Cells plated on either laminin-1 (A–D and F) or collagen I (E) at 37°C for 1 h were processed for double immunofluorescence as described using the rat GoH3 mAb followed by a rhodamine-conjugated anti–rat antibody and FITC-conjugated phalloidin. The confocal images shown represent optical sections of the ventral surface of the cells. (A and C) GoH3. (B, D, E, and F) Phalloidin. A and B demonstrate colocalization of α6β4 and F-actin in a group of filopodia. D shows the formation of actin cables on the top lamella that project into filopodia. These filopodia are enriched in α6β4 (C). E shows the presence of polygonal actin cables in clone A cells plated on collagen I. In F, the cells were incubated with 2B7 antibody for 30 min before fixation. Note the disappearance of actin cables (remaining protrusions are presumably retraction fibers, see text). Bars, 10 μm.
Figure 10
The integrin α6β4 localized at the marginal areas of clone A cells on laminin-1 does not colocalize with cytokeratins. Cells were plated on laminin and incubated for 1 h at 37°C . In A, the cells were fixed immediately after the incubation period in paraformaldehyde and then permeabilized with Triton X-100. In B, cells were extracted first with a Triton X-100 buffer before fixation. In C, the cells were extracted with a Tween-40/deoxycholate buffer before fixation. After fixation, the cells in A–C were stained by a double immunofluorescence protocol using rat GoH3 mAb and a mouse pan-cytokeratin mAb antibody followed by a combination of a TRITC-conjugated anti–rat antibody and a FITC-conjugated anti–mouse antibody that do not cross-react. The ventral surface of the cells was analyzed by confocal microscopy. Red, GoH3; green, cytokeratin; yellow, colocalization. In A and B, the marginal areas (edges of lamellae, filopodia, and retraction fibers) exhibit positive α6β4 staining but no cytokeratin staining. Note in B the persistence of α6β4 staining in marginal clusters arranged in streaks that are likely portions of filopodia. In C, the Tween-40/deoxycholate buffer extracted most of the α6β4 staining in filopodia and lamellae. However, α6β4 staining persisted at the base of lamellae where it colocalized with cytokeratins. The cytokeratin staining was digitally “overexposed” to detect any possible cytokeratin expression. Bar, 10 μm.
Figure 10
The integrin α6β4 localized at the marginal areas of clone A cells on laminin-1 does not colocalize with cytokeratins. Cells were plated on laminin and incubated for 1 h at 37°C . In A, the cells were fixed immediately after the incubation period in paraformaldehyde and then permeabilized with Triton X-100. In B, cells were extracted first with a Triton X-100 buffer before fixation. In C, the cells were extracted with a Tween-40/deoxycholate buffer before fixation. After fixation, the cells in A–C were stained by a double immunofluorescence protocol using rat GoH3 mAb and a mouse pan-cytokeratin mAb antibody followed by a combination of a TRITC-conjugated anti–rat antibody and a FITC-conjugated anti–mouse antibody that do not cross-react. The ventral surface of the cells was analyzed by confocal microscopy. Red, GoH3; green, cytokeratin; yellow, colocalization. In A and B, the marginal areas (edges of lamellae, filopodia, and retraction fibers) exhibit positive α6β4 staining but no cytokeratin staining. Note in B the persistence of α6β4 staining in marginal clusters arranged in streaks that are likely portions of filopodia. In C, the Tween-40/deoxycholate buffer extracted most of the α6β4 staining in filopodia and lamellae. However, α6β4 staining persisted at the base of lamellae where it colocalized with cytokeratins. The cytokeratin staining was digitally “overexposed” to detect any possible cytokeratin expression. Bar, 10 μm.
Figure 11
The integrin α6β4 is associated with F-actin. Cells were plated on laminin and incubated for 1 h at 37°C . In A–C, the cells were extracted first with a Triton X-100 buffer before fixation with paraformaldehyde. In D, the cells were extracted with a Tween-40/deoxycholate buffer before fixation. After fixation, the cells in A and B were double immunostained using rat GoH3 mAb followed by a rhodamine-conjugated anti–rat antibody (A) and FITC-conjugated phalloidin (B). In C, the cells were stained with mouse K-20 mAb antibody followed by an FITC-conjugated anti–mouse antibody. In D, the cells were stained with phalloidin. The ventral area of the cells was analyzed by confocal microscopy. Note in A and B the colocalization of α6β4 (A) and F-actin (B) at the roots of filopodia (arrowheads). Several of these colocalization areas are in continuity with actin cables (B). Bar, 10 μm.
Figure 12
The actin-severing protein gelsolin releases α6β4 integrin from permeabilized clone A cells. Cells were plated on laminin-1 and incubated for 1 h at 37°C. After permeablization with a Triton X-100 buffer, the cells were incubated with either gelsolin (G) or control buffer (C) for 30 min. The gelsolin-liberated fraction was immunoprecipitated with an α6-specific antibody (2B7), subjected to SDS-PAGE, and immunoblotted with a β4-specific polyclonal antibody. An aliquot of the gelsolin-liberated fraction was subjected to SDS-PAGE and stained with Coomassie blue to detect the 43-kD actin band that was evident in the gelsolin-treated but not the control cells.
Figure 13
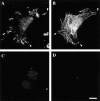
The α6β4 integrin is localized in actin-containing motility structures in other carcinoma cells. MIP-101 (A and B), MDA-MB-231 (C and D), and CCL-228 (E) carcinoma cells were analyzed by double immunostaining with the β4-specific A9 antibody (A, C, and E) and FITC-phalloidin (B and D). Note the concentration of α6β4 in the actin nodes present in the filopodia of MIP-101 cells (A and B, arrowheads) and the distribution of α6β4 in filopodia, retraction fibers, and lamellae of MDA-MB-231 and CCL-228 cells. (F) An α6-specific antibody inhibits formation of lamellae in CCL-228 cells. Cells were plated on laminin-1 in the presence or absence of 2B7 for 1 h. The cells were photographed, and their lamellar area (μm2/cell) was determined by digital image analysis. 50 cells were analyzed for each condition. Error bar represents SEM. Bar, 20 μm.
Figure 13
The α6β4 integrin is localized in actin-containing motility structures in other carcinoma cells. MIP-101 (A and B), MDA-MB-231 (C and D), and CCL-228 (E) carcinoma cells were analyzed by double immunostaining with the β4-specific A9 antibody (A, C, and E) and FITC-phalloidin (B and D). Note the concentration of α6β4 in the actin nodes present in the filopodia of MIP-101 cells (A and B, arrowheads) and the distribution of α6β4 in filopodia, retraction fibers, and lamellae of MDA-MB-231 and CCL-228 cells. (F) An α6-specific antibody inhibits formation of lamellae in CCL-228 cells. Cells were plated on laminin-1 in the presence or absence of 2B7 for 1 h. The cells were photographed, and their lamellar area (μm2/cell) was determined by digital image analysis. 50 cells were analyzed for each condition. Error bar represents SEM. Bar, 20 μm.
Similar articles
- Intestinal restitution: progression of actin cytoskeleton rearrangements and integrin function in a model of epithelial wound healing.
Lotz MM, Rabinovitz I, Mercurio AM. Lotz MM, et al. Am J Pathol. 2000 Mar;156(3):985-96. doi: 10.1016/S0002-9440(10)64966-8. Am J Pathol. 2000. PMID: 10702414 Free PMC article. - RhoA function in lamellae formation and migration is regulated by the alpha6beta4 integrin and cAMP metabolism.
O'Connor KL, Nguyen BK, Mercurio AM. O'Connor KL, et al. J Cell Biol. 2000 Jan 24;148(2):253-8. doi: 10.1083/jcb.148.2.253. J Cell Biol. 2000. PMID: 10648558 Free PMC article. - The alpha 6 beta 4 integrin and epithelial cell migration.
Mercurio AM, Rabinovitz I, Shaw LM. Mercurio AM, et al. Curr Opin Cell Biol. 2001 Oct;13(5):541-5. doi: 10.1016/s0955-0674(00)00249-0. Curr Opin Cell Biol. 2001. PMID: 11544021 Review. - Integrin laminin receptors and breast carcinoma progression.
Mercurio AM, Bachelder RE, Chung J, O'Connor KL, Rabinovitz I, Shaw LM, Tani T. Mercurio AM, et al. J Mammary Gland Biol Neoplasia. 2001 Jul;6(3):299-309. doi: 10.1023/a:1011323608064. J Mammary Gland Biol Neoplasia. 2001. PMID: 11547899 Review.
Cited by
- Force Matters: Biomechanical Regulation of Cell Invasion and Migration in Disease.
Kai F, Laklai H, Weaver VM. Kai F, et al. Trends Cell Biol. 2016 Jul;26(7):486-497. doi: 10.1016/j.tcb.2016.03.007. Epub 2016 Apr 4. Trends Cell Biol. 2016. PMID: 27056543 Free PMC article. Review. - Unique expression pattern of the alpha6beta4 integrin and laminin-5 in human prostate carcinoma.
Davis TL, Cress AE, Dalkin BL, Nagle RB. Davis TL, et al. Prostate. 2001 Feb 15;46(3):240-8. doi: 10.1002/1097-0045(20010215)46:3<240::aid-pros1029>3.0.co;2-0. Prostate. 2001. PMID: 11170153 Free PMC article. - Neuropilin-2 regulates α6β1 integrin in the formation of focal adhesions and signaling.
Goel HL, Pursell B, Standley C, Fogarty K, Mercurio AM. Goel HL, et al. J Cell Sci. 2012 Jan 15;125(Pt 2):497-506. doi: 10.1242/jcs.094433. Epub 2012 Feb 2. J Cell Sci. 2012. PMID: 22302985 Free PMC article. - Integrin β4 regulates SPARC protein to promote invasion.
Gerson KD, Shearstone JR, Maddula VSRK, Seligmann BE, Mercurio AM. Gerson KD, et al. J Biol Chem. 2012 Mar 23;287(13):9835-9844. doi: 10.1074/jbc.M111.317727. Epub 2012 Feb 3. J Biol Chem. 2012. PMID: 22308039 Free PMC article. - ITGB4 as a novel serum diagnosis biomarker and potential therapeutic target for colorectal cancer.
Jiang X, Wang J, Wang M, Xuan M, Han S, Li C, Li M, Sun XF, Yu W, Zhao Z. Jiang X, et al. Cancer Med. 2021 Oct;10(19):6823-6834. doi: 10.1002/cam4.4216. Epub 2021 Aug 20. Cancer Med. 2021. PMID: 34414684 Free PMC article.
References
- Borradori L, Sonnenberg A. Hemidesmosomes—roles in adhesion, signaling and human diseases. Curr Opin Cell Biol. 1996;8:647–656. - PubMed
- Capco DG, Wan KM, Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982;29:847–858. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous