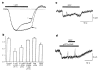Cyclic-nucleotide-gated channels mediate synaptic feedback by nitric oxide - PubMed (original) (raw)
Cyclic-nucleotide-gated channels mediate synaptic feedback by nitric oxide
A Savchenko et al. Nature. 1997 Dec.
Abstract
Cyclic-nucleotide-gated (CNG) channels in outer segments of vertebrate photoreceptors generate electrical signals in response to changes in cyclic GMP concentration during phototransduction. CNG channels also allow the influx of Ca2+, which is essential for photoreceptor adaptation. In cone photoreceptors, cGMP triggers an increase in membrane capacitance indicative of exocytosis, suggesting that CNG channels are also involved in synaptic function. Here we examine whether CNG channels reside in cone terminals and whether they regulate neurotransmitter release, specifically in response to nitric oxide (NO), a retrograde transmitter that increases cGMP synthesis and potentiates synaptic transmission in the brain. Using intact retina, we show that endogenous NO modulates synapses between cones and horizontal cells. In experiments on isolated cones, we show directly that CNG channels occur in clusters and are indirectly activated by S-nitrosocysteine (SNC), an NO donor. Furthermore, both SNC and pCPT-cGMP, a membrane-permeant analogue of cGMP, trigger the release of transmitter from the cone terminals. The NO-induced transmitter release is suppressed by guanylate cyclase inhibitors and prevented by direct activation of CNG channels, indicating that their activation is required for NO to elicit release. These results expand our view of CNG channel function to include the regulation of synaptic transmission and mediation of the presynaptic effects of NO.
Figures
Figure 1
Responses of cone terminals to pCPT–cGMP. a, Acutely dissociated cone photoreceptor from Anolis carolinensis retina. Note the outer segment (OS), oil droplet (OD), inner segment (IS), and presynaptic terminal, or ‘pedicle’, of the cone (T). Scale bar, 10 μm. b, Records from a cone containing a terminal (left) and from a cone lacking a terminal (right). Only the cone with the terminal exhibits an inward current in response to 100 μM pCPT–cGMP (top traces; holding potential = −60 mV). Similarly, only the cone with the terminal exhibits voltage-gated Ca2+ currents in response to 20 mV incremental depolarizations from −60 mV to +40 mV (bottom traces). c, An isolated terminal exhibits current in response to 100 μM pCPT–cGMP and 10 mV incremental depolarizations from −60 to +30 mV. Tail currents result from Ca2+-activated Cl− channels,, owing to the presence of 140 mM Cl− in the pipette, rather than 30 mM Cl− plus 110 mM aspartate, as in b.
Figure 2
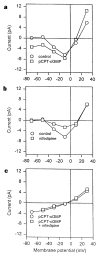
Steady-state I–V curves of the pCPT–cGMP-activated and voltage-gated Ca2+ currents. a, I–V curve in normal saline (control) and after application of 100 μM pCPT–cGMP. b, I–V curve in normal saline and after application of 1 μM nifedipine. c, I–V relation of the pCPT–cGMP-activated conductance obtained by subtraction of the I–V curve in control saline from the I–V curve in pCPT–cGMP, both obtained in the absence (circles) or presence (squares) of 1 μM nifedipine. All currents were measured at the end of 200-ms voltage pulses from a holding potential of −60 mV.
Figure 3
CNG channels in cone terminals. a, Direct activation of CNG channels in an inside-out patch excised from a cone terminal. The patch contained an estimated 29 channels. b, Dose–response curves for cone terminal (t) and outer segment (os) CNG-channel activation by cGMP and pCPT–cGMP. Continuous curves show fits to the Hill equation. c, Distribution of CNG channels within cones.
Figure 4
Modulation of cone synapses by NO. a, Endogenous NO modulates synaptic communication between cones and horizontal cells. Voltage responses from horizontal cells incubated in normal saline (trace 1) or in 2 mM L-NNA (trace 2) to 500-ms steps of full-field illumination (2 × 10−7 μW μm−2). Control and L-NNA responses were normalized to peak amplitudes (15 and 10 mV, respectively). b, Summary of feedback response experiments, showing mean ± s.e.m. of the ‘response sag’, defined as 1 − (S/P), where P is the peak voltage and S is the voltage at the end of the light step. Recordings were made from horizontal cells in retinae super-fused for 1 h with control saline, L-NNA, 2 mM L-arginine, 2 mM L-arginine + L-NNA, or 100 μM ODQ, as indicated. Results with ODQ (P < 0.02) or L-NNA alone (P < 0.001) were statistically different from control. c, Activation of CNG current in an isolated lizard cone terminal by 50 μM SNC. d, Occlusion of the SNC response by prior application of 100 μM pCPT–cGMP.
Figure 5
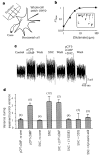
Biosensor measurements of transmitter release from cone terminals. a, Experimental arrangement. b, Sensitivity of the glutamate response of the horizontal cell biosensor. Bath application of 1, 2.5, 5, 10 and 100 μM glutamate elicits the responses shown in the inset (vertical scale 10 pA, horizontal scale 10 s). The graph shows the Hill fit to peak responses with a _K_1/2 of 4.9 μM. c, Steady-state changes in current noise in a horizontal cell contacted by the terminal of a dissociated cone. Recordings show reversible effects of 100 μM pCPT–cGMP and 50 μM SNC applied alone, and pCPT–cGMP and SNC applied together. d, Summary of release experiments. Ratio of horizontal-cell current variance during treatment to control variance. Bars show mean variance ±s.e.m., with number of cells tested in parentheses. Results for pCPT–cGMP, SNC and SNC + pCPT–cGMP were statistically different from control (P < 0.001).
Similar articles
- Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.
Korenbrot JI, Rebrik TI. Korenbrot JI, et al. Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review. - In intact mammalian photoreceptors, Ca2+-dependent modulation of cGMP-gated ion channels is detectable in cones but not in rods.
Rebrik TI, Korenbrot JI. Rebrik TI, et al. J Gen Physiol. 2004 Jan;123(1):63-75. doi: 10.1085/jgp.200308952. J Gen Physiol. 2004. PMID: 14699078 Free PMC article. - A cGMP-gated current can control exocytosis at cone synapses.
Rieke F, Schwartz EA. Rieke F, et al. Neuron. 1994 Oct;13(4):863-73. doi: 10.1016/0896-6273(94)90252-6. Neuron. 1994. PMID: 7946333 - Developmental expression of retinal cone cGMP-gated channels: evidence for rapid turnover and trophic regulation.
Ko GY, Ko ML, Dryer SE. Ko GY, et al. J Neurosci. 2001 Jan 1;21(1):221-9. doi: 10.1523/JNEUROSCI.21-01-00221.2001. J Neurosci. 2001. PMID: 11150339 Free PMC article. - Retinal Cyclic Nucleotide-Gated Channels: From Pathophysiology to Therapy.
Michalakis S, Becirovic E, Biel M. Michalakis S, et al. Int J Mol Sci. 2018 Mar 7;19(3):749. doi: 10.3390/ijms19030749. Int J Mol Sci. 2018. PMID: 29518895 Free PMC article. Review.
Cited by
- Phosphorylation of N-methyl-D-aspartic acid receptor-associated neuronal nitric oxide synthase depends on estrogens and modulates hypothalamic nitric oxide production during the ovarian cycle.
Parkash J, d'Anglemont de Tassigny X, Bellefontaine N, Campagne C, Mazure D, Buée-Scherrer V, Prevot V. Parkash J, et al. Endocrinology. 2010 Jun;151(6):2723-35. doi: 10.1210/en.2010-0007. Epub 2010 Apr 6. Endocrinology. 2010. PMID: 20371700 Free PMC article. - An endogenous RNA transcript antisense to CNG(alpha)1 cation channel mRNA.
Cheng CH, Yew DT, Kwan HY, Zhou Q, Huang Y, Liu Y, Chan WY, Yao X. Cheng CH, et al. Mol Biol Cell. 2002 Oct;13(10):3696-705. doi: 10.1091/mbc.e02-03-0127. Mol Biol Cell. 2002. PMID: 12388767 Free PMC article. - Calcium homeostasis and cone signaling are regulated by interactions between calcium stores and plasma membrane ion channels.
Szikra T, Barabas P, Bartoletti TM, Huang W, Akopian A, Thoreson WB, Krizaj D. Szikra T, et al. PLoS One. 2009 Aug 21;4(8):e6723. doi: 10.1371/journal.pone.0006723. PLoS One. 2009. PMID: 19696927 Free PMC article. - Regulation of structural plasticity by different channel types in rod and cone photoreceptors.
Zhang N, Townes-Anderson E. Zhang N, et al. J Neurosci. 2002 Aug 15;22(16):7065-79. doi: 10.1523/JNEUROSCI.22-16-07065.2002. J Neurosci. 2002. PMID: 12177203 Free PMC article. - C-Linker of cyclic nucleotide-gated channels controls coupling of ligand binding to channel gating.
Paoletti P, Young EC, Siegelbaum SA. Paoletti P, et al. J Gen Physiol. 1999 Jan;113(1):17-34. doi: 10.1085/jgp.113.1.17. J Gen Physiol. 1999. PMID: 9874685 Free PMC article.
References
- Yau KW, Baylor DA. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. - PubMed
- Koutalos Y, Yau KW. Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci. 1996;19:73–81. - PubMed
- Rieke F, Schwartz EA. A cGMP-gated current can control exocytosis at cone synapses. Neuron. 1994;13:863–873. - PubMed
- Garthwaite J, Charles SJ, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous