Activation of p38 mitogen-activated protein kinase by sodium salicylate leads to inhibition of tumor necrosis factor-induced IkappaB alpha phosphorylation and degradation - PubMed (original) (raw)
Activation of p38 mitogen-activated protein kinase by sodium salicylate leads to inhibition of tumor necrosis factor-induced IkappaB alpha phosphorylation and degradation
P Schwenger et al. Mol Cell Biol. 1998 Jan.
Abstract
Many actions of the proinflammatory cytokines tumor necrosis factor (TNF) and interleukin-1 (IL-1) on gene expression are mediated by the transcription factor NF-kappaB. Activation of NF-kappaB by TNF and IL-1 is initiated by the phosphorylation of the inhibitory subunit, IkappaB, which targets IkappaB for degradation and leads to the release of active NF-kappaB. The nonsteroidal anti-inflammatory drug sodium salicylate (NaSal) interferes with TNF-induced NF-kappaB activation by inhibiting phosphorylation and subsequent degradation of the IkappaB alpha protein. Recent evidence indicated that NaSal activates the p38 mitogen-activated protein kinase (MAPK), raising the possibility that inhibition of NF-kappaB activation by NaSal is mediated by p38 MAPK. We now show that inhibition of TNF-induced IkappaB alpha phosphorylation and degradation by NaSal is prevented by treatment of cells with SB203580, a highly specific p38 MAPK inhibitor. Both p38 activation and inhibition of TNF-induced IkappaB alpha degradation were seen after only 30 s to 1 min of NaSal treatment. Induction of p38 MAPK activation and inhibition of TNF-induced IkappaB alpha degradation were demonstrated with pharmacologically achievable doses of NaSal. These findings provide evidence for a role of NaSal-induced p38 MAPK activation in the inhibition of TNF signaling and suggest a possible role for the p38 MAPK in the anti-inflammatory actions of salicylates. In addition, these results implicate the p38 MAPK as a possible negative regulator of TNF signaling that leads to NF-kappaB activation.
Figures
FIG. 1
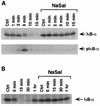
NaSal inhibits TNF-induced IκBα phosphorylation and degradation. (A) COS-1 cells were either treated for 1 h with NaSal (20 mM) or left untreated. They were then either left unstimulated (Ctrl) or stimulated for the indicated times with TNF (20 ng/ml). Lysates were blotted with antibodies against IκBα (top panel) or with antibodies to phosphorylated IκBα (pIκB-α; bottom panel). (B) COS-1 cells were treated as described above, and lysates were blotted with an anti-IκBα antibody.
FIG. 2
Selective inhibition of TNF-induced IκBα degradation by NaSal. COS-1 cells were preincubated for 1 h in the presence (+) or absence (−) of NaSal (20 mM). They were then left untreated (Ctrl) or treated with TNF (20 ng/ml) or IL-1 (4 ng/ml) for 15 min. In the last two lanes, an initial 15-min TNF treatment was immediately followed by a 15-min IL-1 treatment. Lysates were blotted with anti-IκBα antibody.
FIG. 3
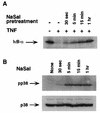
Inhibition of TNF-induced IκBα phosphorylation and degradation by NaSal is prevented by SB203580. (A) COS-1 and HT-29 cells were preincubated for 1.5 h in the presence (+) or absence (−) of SB203580 (10 μM). The cells were then incubated for 1 h in the presence or absence of NaSal (20 mM) and subsequently incubated for 5 min in the presence or absence of TNF (20 ng/ml). Lysates were blotted with antibodies against phosphorylated IκBα (pIκB-α; top panel) and with antibodies to IκBα (bottom panel). (B) COS-1 and HT-29 cells were treated as described above except that the duration of TNF treatment was 15 min instead of 5 min. Lysates were blotted with anti-IκBα antibody.
FIG. 4
Kinetics of inhibition of IκBα degradation and of p38 MAPK activation by NaSal. (A) COS-1 cells were either left untreated (−) or treated for the indicated times with NaSal (20 mM) and then stimulated for 15 min with TNF (20 ng/ml). Lysates were blotted with anti-IκBα antibody. (B) COS-1 cells were treated for the indicated times with NaSal (20 mM) alone, lysed, and blotted with anti-phospho-p38 MAPK antibody (pp38; top panel) and with anti-p38 MAPK antibody (p38; bottom panel).
FIG. 5
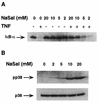
Inhibition of IκBα degradation and induction of p38 MAPK activation by different doses of NaSal. (A) COS-1 cells were treated for 15 min with the indicated doses of NaSal and then left unstimulated (−) or stimulated (+) for 15 min with TNF (20 ng/ml). Lysates were blotted with anti-IκBα antibody. (B) COS-1 cells were treated for 15 min with the indicated doses of NaSal alone, lysed, and blotted with anti-phospho-p38 MAPK antibody (pp38; top panel) and with anti-p38 MAPK antibody (p38; bottom panel).
FIG. 6
Kinetics of p38 MAPK activation by NaSal and TNF. COS-1 cells were either left untreated (Ctrl) or treated for the indicated times with TNF (20 ng/ml) or NaSal (20 mM). Lysates were blotted with anti-phospho-p38 MAPK antibody (pp38; top panel) and with anti-p38 MAPK antibody (p38; bottom panel).
Similar articles
- Cell stress and MKK6b-mediated p38 MAP kinase activation inhibit tumor necrosis factor-induced IkappaB phosphorylation and NF-kappaB activation.
Alpert D, Schwenger P, Han J, Vilcek J. Alpert D, et al. J Biol Chem. 1999 Aug 6;274(32):22176-83. doi: 10.1074/jbc.274.32.22176. J Biol Chem. 1999. PMID: 10428782 - Activation of p38 mitogen-activated protein kinase and nuclear factor-kappaB in tumour necrosis factor-induced eotaxin release of human eosinophils.
Wong CK, Zhang JP, Ip WK, Lam CW. Wong CK, et al. Clin Exp Immunol. 2002 Jun;128(3):483-9. doi: 10.1046/j.1365-2249.2002.01880.x. Clin Exp Immunol. 2002. PMID: 12067303 Free PMC article. - Intracellular signaling in rat cultured vascular smooth muscle cells: roles of nuclear factor-kappaB and p38 mitogen-activated protein kinase on tumor necrosis factor-alpha production.
Yamakawa T, Eguchi S, Matsumoto T, Yamakawa Y, Numaguchi K, Miyata I, Reynolds CM, Motley ED, Inagami T. Yamakawa T, et al. Endocrinology. 1999 Aug;140(8):3562-72. doi: 10.1210/endo.140.8.6914. Endocrinology. 1999. PMID: 10433212 - Inhibition of IkappaB kinase activity by sodium salicylate in vitro does not reflect its inhibitory mechanism in intact cells.
Alpert D, Vilcek J. Alpert D, et al. J Biol Chem. 2000 Apr 14;275(15):10925-9. doi: 10.1074/jbc.275.15.10925. J Biol Chem. 2000. PMID: 10753891 - Activation of the AP-1 transcription factor by inflammatory cytokines of the TNF family.
Kyriakis JM. Kyriakis JM. Gene Expr. 1999;7(4-6):217-31. Gene Expr. 1999. PMID: 10440223 Free PMC article. Review.
Cited by
- Raf-MEK-Erk cascade in anoikis is controlled by Rac1 and Cdc42 via Akt.
Zugasti O, Rul W, Roux P, Peyssonnaux C, Eychene A, Franke TF, Fort P, Hibner U. Zugasti O, et al. Mol Cell Biol. 2001 Oct;21(19):6706-17. doi: 10.1128/MCB.21.19.6706-6717.2001. Mol Cell Biol. 2001. PMID: 11533257 Free PMC article. - TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells.
Pan Z, Wang Z, Yang H, Zhang F, Reinach PS. Pan Z, et al. Invest Ophthalmol Vis Sci. 2011 Jan 21;52(1):485-93. doi: 10.1167/iovs.10-5801. Print 2011 Jan. Invest Ophthalmol Vis Sci. 2011. PMID: 20739465 Free PMC article. - (E)-2,4-bis(p-hydroxyphenyl)-2-butenal has an antiproliferative effect on NSCLC cells induced by p38 MAPK-mediated suppression of NF-κB and up-regulation of TNFRSF10B (DR5).
Kollipara PS, Jeong HS, Han SB, Hong JT. Kollipara PS, et al. Br J Pharmacol. 2013 Mar;168(6):1471-84. doi: 10.1111/bph.12024. Br J Pharmacol. 2013. PMID: 23082969 Free PMC article. - Aspirin induces IL-4 production: augmented IL-4 production in aspirin-exacerbated respiratory disease.
Kong SK, Soo Kim B, Gi Uhm T, Soo Chang H, Sook Park J, Woo Park S, Park CS, Chung IY. Kong SK, et al. Exp Mol Med. 2016 Jan 8;48(1):e202. doi: 10.1038/emm.2015.96. Exp Mol Med. 2016. PMID: 27534531 Free PMC article. - Shared and Related Molecular Targets and Actions of Salicylic Acid in Plants and Humans.
Ding Y, Fan B, Zhu C, Chen Z. Ding Y, et al. Cells. 2023 Jan 4;12(2):219. doi: 10.3390/cells12020219. Cells. 2023. PMID: 36672154 Free PMC article. Review.
References
- Abramson S B, Weissmann G. The mechanisms of action of nonsteroidal antiinflammatory drugs. Arthritis Rheum. 1989;32:1–9. - PubMed
- Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. - PubMed
- Baldwin A S., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. - PubMed
- Beauparlant P, Hiscott J. Biological and biochemical inhibitors of the NF-κB/Rel proteins and cytokine synthesis. Cytokine Growth Factor Rev. 1996;7:175–190. - PubMed
- Beg A A, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 CA009161/CA/NCI NIH HHS/United States
- 5T32-CA09161/CA/NCI NIH HHS/United States
- T32 GM007308/GM/NIGMS NIH HHS/United States
- R35CA42568/CA/NCI NIH HHS/United States
- 5T32-GM07308/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources