Structural determinants of SHP-2 function and specificity in Xenopus mesoderm induction - PubMed (original) (raw)
Structural determinants of SHP-2 function and specificity in Xenopus mesoderm induction
A M O'Reilly et al. Mol Cell Biol. 1998 Jan.
Abstract
SHP-2 is a positive component of many receptor tyrosine kinase signaling pathways. The related protein-tyrosine phosphatase (PTP) SHP-1 usually acts as a negative regulator. The precise domains utilized by SHP-2 to transmit positive signals in vivo and the basis for specificity between SHP-1 and SHP-2 are not clear. In Xenopus, SHP-2 is required for mesoderm induction and completion of gastrulation. We investigated the effects of SHP-2 mutants and SHP-2/SHP-1 chimeras on basic fibroblast growth factor-induced mesoderm induction. Both SH2 domains and the PTP domain are required for normal SHP-2 function in this pathway. The N-terminal SH2 domain is absolutely required, whereas the C-terminal SH2 contributes to wild-type function. The C-terminal tyrosyl phosphorylation sites and proline-rich region are dispensable, arguing against adapter models of SHP-2 function. Although the SH2 domains contribute to SHP-2 specificity, studies of SHP chimeras reveal that substantial specificity resides in the PTP domain. Thus, PTP domains exhibit biologically relevant specificity in vivo, and noncatalytic and catalytic domains of PTPs contribute to specificity in a combinatorial fashion.
Figures
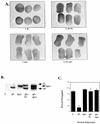
FIG. 1
Microinjection constructs: schematic representations of human SHP-2 and chimeric cDNA clones showing functional domains that might participate in bFGF signaling, including the two SH2 domains, the SH2-PTP linker, the PTP domain, the C-terminal tyrosine phosphorylation sites, and the proline-rich region. Amino acid numbers corresponding to human SHP-2 (24) are indicated above the diagram. (A) Full-length SHP-2 and ΔP, a mutant with a 31-amino-acid deletion in the PTP domain, which acts as a dominant negative mutant. (B) SH2 domain mutants with point mutations in the essential βb5 arginine of both SH2 domains (R32,138K) or individual N-SH2 (R32K) or C-SH2 (R138K) domains in the context of WT SHP-2 or ΔP, as indicated. (C) C-terminal tail mutants with tyrosine (Y)-to-phenylalanine (F) mutations at position 542 and/or 580 in the context of WT SHP-2 or ΔP, as indicated. Δpro, 10-amino-acid deletion of the proline-rich region between the two tyrosines. (D) Chimeras between SHP-2 and SHP-1. SHP-1 domains (white boxes) and SHP-2 domains (black boxes) are indicated. Shown are SHP-1; the 21 chimera, containing the SH2 domains and linker region of SHP-2 fused to the PTP and C-terminal tail of SHP-1; the 212 chimera, containing the SH2 domains and linker of SHP-2 fused to the PTP domain of SHP-1 and the C-terminal tail of SHP-2; and the 12 chimera, containing the SH2 domains of SHP-1 fused to the linker, PTP domain, and C-terminal tail of SHP-2.
FIG. 2
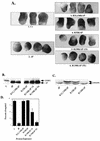
SH2 domains are required for ΔP to act as a dominant negative mutant. Representative experimental results show animal cap elongation, protein levels, and MAPK activation following injection of the indicated SHP-2 mutants. (A) bFGF-stimulated animal caps at stage 10.5. The injected mRNA is indicated beneath each panel. C+, uninjected animal caps stimulated for 2 h with bFGF (100 ng/ml). These caps are elongated. In contrast, ΔP-injected caps (ΔP) show no elongation and are scored as blocked. 1× and 5×, concentrations used (see Materials and Methods). (B) Immunoblot analysis of injected animal caps at stage 8. Total lysates of animal caps were probed with anti-PTP1D/SHP-2 monoclonal antibodies (Transduction Laboratories). ΔP proteins are indicated (lower band). This antibody cross-reacts with endogenous Xenopus SHP-2 (XSHP2), which serves as a loading control. The injected mRNA is indicated beneath each lane. (C) MAPK activation in animal caps stimulated with bFGF (100 ng/ml) at 25°C for 5 min. Total lysates of animal caps were probed with anti-Xenopus MAPK (anti-XMAPK antibodies) (see Materials and Methods). The injected mRNA is indicated beneath each lane. The amount of R138/ΔP injected here is equivalent to 5× in panel A. (D) Quantitation of animal cap elongation. Mean percentages of elongated caps are shown. Error bars show the standard error of the mean for each injected mRNA. Injected mRNA is indicated beneath each lane. Percentages are based on a minimum of 45 total animal caps in three or more separate experiments.
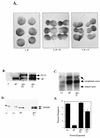
FIG. 3
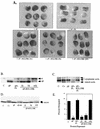
Intact SH2 domains are required for SHP-2 to rescue the ΔP-induced block of bFGF signaling. Representative experimental results show elongation, protein levels, muscle actin induction, and MAPK activation in response to bFGF stimulation of animal caps from embryos injected with the indicated RNAs. (A) bFGF-stimulated animal caps at stage 10.5 were analyzed as for Fig. 2A. Shown are caps from embryos injected with ΔP alone (ΔP), with ΔP plus WT SHP-2 (ΔP + FL), or with ΔP plus the indicated increasing amounts of the R32,138K mutant (1×, 5×, and 10×). (B) Immunoblot analysis of the injected animal caps from panel A. (C) Northern blot analysis of induction of the mesoderm-specific marker muscle actin. Caps collected at stage 21 were analyzed for expression of muscle actin (lowest band). The two upper bands represent cytoplasmic actin, which cross-reacts with the probe and acts as a loading control for the experiment. The injected mRNA is indicated beneath each lane. (D) MAPK activation in animal caps injected with the indicated constructs, monitored as described for Fig. 2D. XMAPK, Xenopus MAPK. (E) Quantitation of animal cap elongation, as described for Fig. 2D. The injected mRNA is indicated beneath each lane. Percentages are based on a minimum of 30 total animal caps in three separate experiments.
FIG. 4
Tyrosine-to-phenylalanine mutations do not affect the ability of ΔP to block bFGF signaling. Representative experimental results show animal cap elongation, protein levels, and MAPK activation. (A) bFGF-stimulated animal caps at stage 10.5, prepared from embryos injected with the indicated mRNA. (B) Immunoblot analysis, carried out as described for Fig. 2B. Positions of ΔP proteins and WT full-length SHP-2 (FL) are indicated. In this exposure, endogenous Xenopus SHP-2 levels are too low to be detected. The injected mRNA is indicated beneath each lane. (C) MAPK activation in animal caps assayed as described for Fig. 2C. The injected mRNA is indicated beneath each lane. XMAPK, Xenopus MAPK. (D) Quantitation of animal cap elongation, as for Fig. 2D. The injected mRNA is indicated beneath each lane. Percentages are based on a minimum of 45 total animal caps in three or more separate experiments.
FIG. 4
Tyrosine-to-phenylalanine mutations do not affect the ability of ΔP to block bFGF signaling. Representative experimental results show animal cap elongation, protein levels, and MAPK activation. (A) bFGF-stimulated animal caps at stage 10.5, prepared from embryos injected with the indicated mRNA. (B) Immunoblot analysis, carried out as described for Fig. 2B. Positions of ΔP proteins and WT full-length SHP-2 (FL) are indicated. In this exposure, endogenous Xenopus SHP-2 levels are too low to be detected. The injected mRNA is indicated beneath each lane. (C) MAPK activation in animal caps assayed as described for Fig. 2C. The injected mRNA is indicated beneath each lane. XMAPK, Xenopus MAPK. (D) Quantitation of animal cap elongation, as for Fig. 2D. The injected mRNA is indicated beneath each lane. Percentages are based on a minimum of 45 total animal caps in three or more separate experiments.
FIG. 5
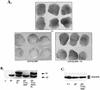
Mutation of tyrosines to phenylalanines does not affect the ability of full-length SHP-2 to rescue the ΔP block. (A) bFGF-stimulated animal caps at stage 10.5 from embryos injected with the indicated mRNA. Shown are caps injected with ΔP alone (ΔP), ΔP plus WT full-length SHP-2 (ΔP + FL), and ΔP plus the indicated single or double tyrosyl phosphorylation site mutants. (B) Immunoblot analysis of SHP-2 expression, as described for Fig. 2B. (C) Quantitation of animal cap elongation, as for Fig. 2D. Percentages are based on a minimum of 70 total animal caps in three or more separate experiments.
FIG. 6
Deletion of the C-terminal prolines has no effect on the ability of SHP-2 to rescue the ΔP block. Representative experimental results show animal cap elongation and protein levels. (A) bFGF-stimulated animal caps from embryos injected with the indicated mRNAs. (B) Immunoblot analysis, as described for Fig. 2B. (C) Quantitation of animal cap elongation, as for Fig. 2D. Percentages are based on a minimum of 37 total animal caps in two or more separate experiments.
FIG. 7
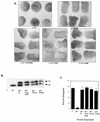
Chimeras containing the PTP domain of SHP-1 act as dominant negative mutants in the animal cap assay. Representative experimental results show animal cap elongation, protein levels, muscle actin induction, and MAPK activation. (A) bFGF-stimulated animal caps at stage 10.5 from embryos injected with either ΔP or the 21 chimera. Note that the two constructs block elongation equivalently. (B) Immunoblot analysis as described for Fig. 2B and MAPK activation in animal caps, assessed as for Fig. 2C. The injected mRNA is indicated beneath each lane. XMAPK, Xenopus MAPK. (C) bFGF-stimulated animal caps at stage 10.5. Shown are caps from embryos injected with ΔP alone (ΔP), ΔP plus WT full-length SHP-2 (ΔP + FL), the 212 chimera alone (212), and the 212 chimera plus WT full-length SHP-2 (212 + FL). (D) Immunoblot analysis as in Fig. 2B. (E) Northern blot analysis of muscle actin mRNA induction, carried out as for Fig. 3C. No muscle actin band is seen in either the ΔP or the 212 lane, even upon longer exposure. (F) MAPK activation in animal caps, as in Fig. 2C. The injected mRNA is indicated beneath each lane. (G) Quantitation of animal cap elongation, as in Fig. 2D. Percentages are based on a minimum of 45 total animal caps in three or more separate experiments.
FIG. 7
Chimeras containing the PTP domain of SHP-1 act as dominant negative mutants in the animal cap assay. Representative experimental results show animal cap elongation, protein levels, muscle actin induction, and MAPK activation. (A) bFGF-stimulated animal caps at stage 10.5 from embryos injected with either ΔP or the 21 chimera. Note that the two constructs block elongation equivalently. (B) Immunoblot analysis as described for Fig. 2B and MAPK activation in animal caps, assessed as for Fig. 2C. The injected mRNA is indicated beneath each lane. XMAPK, Xenopus MAPK. (C) bFGF-stimulated animal caps at stage 10.5. Shown are caps from embryos injected with ΔP alone (ΔP), ΔP plus WT full-length SHP-2 (ΔP + FL), the 212 chimera alone (212), and the 212 chimera plus WT full-length SHP-2 (212 + FL). (D) Immunoblot analysis as in Fig. 2B. (E) Northern blot analysis of muscle actin mRNA induction, carried out as for Fig. 3C. No muscle actin band is seen in either the ΔP or the 212 lane, even upon longer exposure. (F) MAPK activation in animal caps, as in Fig. 2C. The injected mRNA is indicated beneath each lane. (G) Quantitation of animal cap elongation, as in Fig. 2D. Percentages are based on a minimum of 45 total animal caps in three or more separate experiments.
FIG. 8
The ΔP block is partially rescued by coexpression of a chimera containing the PTP domain of SHP-2. Representative experimental results show animal cap elongation, protein levels, muscle actin induction, and MAPK activation. (A) bFGF-stimulated animal caps at stage 10.5 from embryos injected with the indicated mRNAs. Note that ΔP is fully rescued by coexpression of WT full-length SHP-2 (ΔP + FL) and partially rescued upon coexpression of the 12 chimera (ΔP + 12). (B) Immunoblot analysis carried out as for Fig. 2B, except that an antibody directed against the C terminus of SHP-2 (Santa Cruz Biotechnology) was used to allow comparison of the levels of full-length SHP-2, ΔP, and the 12 chimera protein, which share the C terminus of SHP-2 (see Materials and Methods for details). (C) Northern blot analysis of induction of muscle actin mRNA, as for Fig. 3C. (D) MAPK activation in animal caps injected with the indicated constructs, analyzed as for Fig. 2C. XMAPK, Xenopus MAPK. (E) Quantitation of animal cap elongation, as in Fig. 2D. Percentages are based on a minimum of 88 total animal caps in three or more separate experiments.
Similar articles
- Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2.
Saxton TM, Henkemeyer M, Gasca S, Shen R, Rossi DJ, Shalaby F, Feng GS, Pawson T. Saxton TM, et al. EMBO J. 1997 May 1;16(9):2352-64. doi: 10.1093/emboj/16.9.2352. EMBO J. 1997. PMID: 9171349 Free PMC article. - Specificity of the SH2 domains of SHP-1 in the interaction with the immunoreceptor tyrosine-based inhibitory motif-bearing receptor gp49B.
Wang LL, Blasioli J, Plas DR, Thomas ML, Yokoyama WM. Wang LL, et al. J Immunol. 1999 Feb 1;162(3):1318-23. J Immunol. 1999. PMID: 9973385 - Shp-2 tyrosine phosphatase: signaling one cell or many.
Feng GS. Feng GS. Exp Cell Res. 1999 Nov 25;253(1):47-54. doi: 10.1006/excr.1999.4668. Exp Cell Res. 1999. PMID: 10579910 Review. - Regulation of B cell signal transduction by SH2-containing protein-tyrosine phosphatases.
Siminovitch KA, Neel BG. Siminovitch KA, et al. Semin Immunol. 1998 Aug;10(4):329-47. doi: 10.1006/smim.1998.0125. Semin Immunol. 1998. PMID: 9695189 Review.
Cited by
- Crystal structure of human protein tyrosine phosphatase SHP-1 in the open conformation.
Wang W, Liu L, Song X, Mo Y, Komma C, Bellamy HD, Zhao ZJ, Zhou GW. Wang W, et al. J Cell Biochem. 2011 Aug;112(8):2062-71. doi: 10.1002/jcb.23125. J Cell Biochem. 2011. PMID: 21465528 Free PMC article. - Noonan syndrome/leukemia-associated gain-of-function mutations in SHP-2 phosphatase (PTPN11) enhance cell migration and angiogenesis.
Wang S, Yu WM, Zhang W, McCrae KR, Neel BG, Qu CK. Wang S, et al. J Biol Chem. 2009 Jan 9;284(2):913-20. doi: 10.1074/jbc.M804129200. Epub 2008 Nov 13. J Biol Chem. 2009. PMID: 19008228 Free PMC article. - Protein Tyrosine Phosphatase SHP-2 (PTPN11) in Hematopoiesis and Leukemogenesis.
Liu X, Qu CK. Liu X, et al. J Signal Transduct. 2011;2011:195239. doi: 10.1155/2011/195239. Epub 2011 Jun 7. J Signal Transduct. 2011. PMID: 21799948 Free PMC article. - Substrate specificity of protein tyrosine phosphatases 1B, RPTPα, SHP-1, and SHP-2.
Ren L, Chen X, Luechapanichkul R, Selner NG, Meyer TM, Wavreille AS, Chan R, Iorio C, Zhou X, Neel BG, Pei D. Ren L, et al. Biochemistry. 2011 Mar 29;50(12):2339-56. doi: 10.1021/bi1014453. Epub 2011 Feb 18. Biochemistry. 2011. PMID: 21291263 Free PMC article. - Low-dose dasatinib rescues cardiac function in Noonan syndrome.
Yi JS, Huang Y, Kwaczala AT, Kuo IY, Ehrlich BE, Campbell SG, Giordano FJ, Bennett AM. Yi JS, et al. JCI Insight. 2016 Dec 8;1(20):e90220. doi: 10.1172/jci.insight.90220. JCI Insight. 2016. PMID: 27942593 Free PMC article.
References
- Allard J D, Chang H C, Herbst R, McNeill H, Simon M A. The SH2-containing tyrosine phosphatase corkscrew is required during signaling by sevenless, Ras1 and Raf. Development. 1996;122:1137–1146. - PubMed
- Amaya E, Musci T J, Kirschner M W. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–260. - PubMed
- Amaya E, Stein P A, Musci T J, Kirschner M W. FGF signalling in the early specification of mesoderm in Xenopus. Development. 1993;118:477–487. - PubMed
- Aroian R V, Koga M, Mendel J E, Ohshima Y, Sternberg P W. The let-23 gene necessary for Caenorhabditis elegans vulval induction encodes a tyrosine kinase of the EGF receptor subfamily. Nature. 1990;348:693–699. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources