Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication - PubMed (original) (raw)
Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication
A B Niculescu 3rd et al. Mol Cell Biol. 1998 Jan.
Erratum in
- Mol Cell Biol 1998 Mar;18(3):1763
Abstract
It has been proposed that the functions of the cyclin-dependent kinase inhibitors p21(Cip1/Waf1) and p27Kip1 are limited to cell cycle control at the G1/S-phase transition and in the maintenance of cellular quiescence. To test the validity of this hypothesis, p21 was expressed in a diverse panel of cell lines, thus isolating the effects of p21 activity from the pleiotropic effects of upstream signaling pathways that normally induce p21 expression. The data show that at physiological levels of accumulation, p21, in addition to its role in negatively regulating the G1/S transition, contributes to regulation of the G2/M transition. Both G1- and G2-arrested cells were observed in all cell types, with different preponderances. Preponderant G1 arrest in response to p21 expression correlated with the presence of functional pRb. G2 arrest was more prominent in pRb-negative cells. The arrest distribution did not correlate with the p53 status, and proliferating-cell nuclear antigen (PCNA) binding activity of p21 did not appear to be involved, since p27, which lacks a PCNA binding domain, produced similar arrest distributions [corrected], DNA endoreduplication occurred in pRb-negative but not in pRb-positive cells, suggesting that functional pRb is necessary to prevent DNA replication in p21 G2-arrested cells. These results suggest that the primary target of the Cip/Kip family of inhibitors leading to efficient G1 arrest as well as to blockade of DNA replication from either G1 or G2 phase is the pRb regulatory system. Finally, the tendency of Rb-negative cells to undergo endoreduplication cycles when p21 is expressed may have negative implications in the therapy of Rb-negative cancers with genotoxic agents that activate the p53/p21 pathway.
Figures
FIG. 1
Tetracycline-controlled expression of p21 in a panel of pRb-positive and pRb-negative cell lines. Shown are Western blots of total-cell lysates (0.2 mg of protein) with an antibody against p21 (C-19). Tetracycline-controlled p21 expression in uninduced cells (lanes C) and induced cells (lanes p21) is demonstrated. Equal numbers of cells were plated at low density (see Materials and Methods) and incubated for 3 days in medium with or without tetracycline. (A) Comparison of the levels of expression in Saos2 Tet p21 and RKO Tet p21 cells. (B) Comparison of the levels of expression in HeLa Tet p21 cells and RKO Tet p21 cells. (C) Comparison of the levels of expression in RKO Tet p21 cells and in RKO cells subjected to 0 or 4 Gy of ionizing radiation. (D) Comparison of the levels of expression in Rat1 Tet p21 cells and the levels in WI38 normal human diploid fibroblasts approaching senescence. (E) Comparison of the levels of expression in H1299 Tet p21 and adenovirus (Ad)-transduced H1299 cells (with an MOI of 200).
FIG. 2
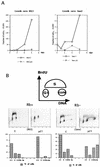
p21 expression arrests cells not only in G1 but also in G2. (A) Growth curves for the p21-transfected RKO and Saos2 cells. Cells were plated at equal densities in triplicate in six-well plates and grown for 6 days in the presence or absence of tetracycline. At the indicated times, the cells were harvested by trypsinization and counted with a hemocytometer. (B) Two-dimensional flow cytometry data of representative experiments illustrating the presence of both G1 and G2/M arrest in response to p21 expression, as well as the different relative preponderance of the two modes of arrest in pRb-positive (RKO) and pRb-negative (Saos2) cells. The cells were grown for 3 days with or without tetracycline. The DNA content measured by propidium iodide staining is shown on the x axis, and BrdU incorporation detected with an FITC-conjugated anti-BrdU antibody is shown on the y axis. The cartoon on top illustrates the distribution of cell cycle phases in such an analysis. The histograms under each flow cytometry plot depict the percentage of cells with G1 (2_n_), S, G2/M (4_n_), and more than 4_n_ DNA content, respectively. Note the presence of a significant population of cells with greater than 4_n_ DNA content in the pRb-negative Saos2 cells. (C and E) Quantitative evaluation of the cell cycle distribution following p21 expression in Rb-negative cells versus Rb-positive cells. The cells were grown for 3 days with or without tetracycline, and their cell cycle distribution was assessed by flow cytometry, as described in Materials and Methods. (C) G1 accumulation; (E) G2/M accumulation. The results are presented as an increase of the ratio of the number of cells with 4_n_ or more DNA content to the number of cells with S-phase DNA content. (D and F) Comparison of the effects of p21 and p27 expression on cell cycle distribution, using adenovirus (Ad) transduction as described in Materials and Methods. MOIs of 100 and 200 were used, as indicated. Flow cytometry data were used for quantitation, as described for panels C and E.
FIG. 2
p21 expression arrests cells not only in G1 but also in G2. (A) Growth curves for the p21-transfected RKO and Saos2 cells. Cells were plated at equal densities in triplicate in six-well plates and grown for 6 days in the presence or absence of tetracycline. At the indicated times, the cells were harvested by trypsinization and counted with a hemocytometer. (B) Two-dimensional flow cytometry data of representative experiments illustrating the presence of both G1 and G2/M arrest in response to p21 expression, as well as the different relative preponderance of the two modes of arrest in pRb-positive (RKO) and pRb-negative (Saos2) cells. The cells were grown for 3 days with or without tetracycline. The DNA content measured by propidium iodide staining is shown on the x axis, and BrdU incorporation detected with an FITC-conjugated anti-BrdU antibody is shown on the y axis. The cartoon on top illustrates the distribution of cell cycle phases in such an analysis. The histograms under each flow cytometry plot depict the percentage of cells with G1 (2_n_), S, G2/M (4_n_), and more than 4_n_ DNA content, respectively. Note the presence of a significant population of cells with greater than 4_n_ DNA content in the pRb-negative Saos2 cells. (C and E) Quantitative evaluation of the cell cycle distribution following p21 expression in Rb-negative cells versus Rb-positive cells. The cells were grown for 3 days with or without tetracycline, and their cell cycle distribution was assessed by flow cytometry, as described in Materials and Methods. (C) G1 accumulation; (E) G2/M accumulation. The results are presented as an increase of the ratio of the number of cells with 4_n_ or more DNA content to the number of cells with S-phase DNA content. (D and F) Comparison of the effects of p21 and p27 expression on cell cycle distribution, using adenovirus (Ad) transduction as described in Materials and Methods. MOIs of 100 and 200 were used, as indicated. Flow cytometry data were used for quantitation, as described for panels C and E.
FIG. 3
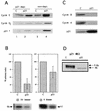
Effects of p21 expression on cyclin-dependent kinase activities, cyclin protein levels and Rb phosphorylation in pRb-positive cells (RKO cells). (A) p21 association with cyclins D and E as analyzed by p21 immunodepletion followed by immunoblotting. Lanes 3 and 4, total-cell lysate; lanes 1 and 2, an equivalent amount of supernatant after one round of p21 immunoprecipitation. (B) p21-mediated changes in cyclin D1- and cyclin E-associated kinase activities. pRb was used as a substrate for cyclin D1-associated kinase, and histone H1 was used as a substrate for cyclin E-associated kinase. Quantitation was performed by PhosphorImager analysis. Data from three experiments are combined in the histograms as the mean ± standard error. A representative autoradiogram is shown below each histogram. (C) Changes in levels of cyclin A and B1 in response to p21 expression. (D) Western blot depicting the effects of p21 expression on pRb phosphorylation. Rb, hypophosphorylated form; P-Rb, hyperphosphorylated form.
FIG. 4
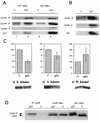
Effects of p21 expression on cyclin-dependent kinase activities and cyclin levels in Rb-negative cells (Saos2 cells). (A) p21 association with cyclin E and A but not cyclin B1. Lanes 3 and 4, total cell lysate; lanes 1 and 2, an equivalent amount of supernatant after one round of p21 immunoprecipitation. (B) Changes in cyclin levels in a parallel experiment; a higher exposure of the film was used to allow the visualization of the low levels present in the control cells. (C) Changes in relevant cyclin-dependent kinase activities, with histone H1 as a substrate. Data from three experiments are combined in the histograms as the mean ± standard error. A representative autoradiogram is shown below each histogram. (D) Western blot depicting the effects of p21 expression on cdc2 levels and phosphorylation status in total cell lysates (non-depl.), cyclin B depleted lysates (cycB depl.), and cyclin B immunoprecipitates (IP cycB). cdc2, dephosphorylated form; cdc2-P, hyperphosphorylated form. Experiments were done as described in Materials and Methods.
FIG. 5
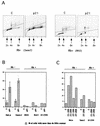
Endoreduplication occurs in pRb-negative cells, but is prevented in pRb-positive cells. (A) Flow cytometric data from representative experiments, illustrating endoreduplication in pRb-negative cells (Saos2 cells) upon p21 expression versus the absence of endoreduplication in pRb-positive cells (RKO cells). Experiments were done as described in the legend to Fig. 2 and in Materials and Methods. Plots of the propidium iodide fluorescence peak area (on abscissa), which measures the DNA content, versus the peak width (on the ordinate), which permits the gating out of clumped cells, are shown. (B) Quantitative evaluation of endoreduplication following p21 expression in pRb-negative and pRb-positive cells. Flow cytometric data were used for quantitation. (C) Comparison of the effects of p21 and p27 on endoreduplication, using adenovirus transduction, as described in Materials and Methods. MOIs of 100 and 200 were used, as indicated. Flow cytometry was used for quantitation.
FIG. 6
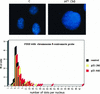
(A) FISH analysis of Saos2 cells with a Chromosome 8 centromeric probe. Images were taken with a 63× oil immersion objective, as described in Materials and Methods. Preparations of nuclei were obtained by hypotonic lysis of cells. Chromatin is stained blue with 4′,6-diamidino-2-phenylindole (DAPI), and the Cy3 fluorescent centromeric probe hybridized to its target appears as pink dots. The left panel shows nuclei from uninduced control cells; the right panel shows an example of a nucleus with an increased number of dots after 3 days of p21 expression. (B) Scoring of a time course experiment. A total of 100 nuclei in each sample, examined with a 63× objective, were scored for the number of dots.
FIG. 7
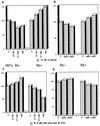
Effects of Cip/Kip expression and γ-irradiation on the cell cycle distribution of MEFs heterozygous (+/−) or homozygous (−/−) for Rb deletion. (A and C) Comparison of effects of γ-irradiation (rad) and p27 adenovirus on the cell cycle distribution in Rb−/− MEFs (Rb−) and Rb+/− MEFs (Rb+). The percentage of cells in G1 (A), and cells with more than 4_n_ DNA content (C) were evaluated by flow cytometry as described in Materials and Methods. Control adenovirus (C) and p27 adenovirus at MOIs of 50 and 100 (27-50 and 27-100) were used as indicated and as described in Materials and Methods and plotted as a percentage of the control. γ-Irradiation consisted of a 4-Gy dose, followed by a 2-Gy dose at 12 h and harvesting at 30 h. (B and D) Similarity of the effects of p21 and p27 adenoviruses (Ad21 and Ad27) on the cell cycle distribution of Rb− and Rb+ MEFs. Equal doses (50 MOI) of the different adenoviruses were used.
FIG. 8
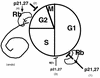
Model of the cell cycle effects of p21 (and p27). Based on differences between pRb-positive and pRb-negative cells, it appears that the presence of a functional Rb pathway is necessary for maximal G1 arrest (pathway 1). G1 arrest in the absence of a functional pRb pathway is inefficient (pathway 3). A second target of p21 action, present in all cell types but more apparent in pRb-negative cells, exists at the G2/M transition (pathway 2). Finally, p21, which probably contributes to preventing inappropriate DNA replication in G2-arrested cells in the presence of a functional pRb pathway (pathway 4), can actually lead to endoreduplication in pRb-negative cells (endo). The cell cycle distributions in our Rb+ and Rb− cells suggest that the Rb-dependent G1/S arrest (pathway 1) is the most sensitive to p21 and is triggered first, followed by the G2/M arrest (pathway 2) and possibly last by an Rb-independent G1/S arrest (pathway 3). Cycling cells will accumulate predominantly in the first of the arrests that comes into effect.
Similar articles
- [Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].
Viallard JF, Lacombe F, Belloc F, Pellegrin JL, Reiffers J. Viallard JF, et al. Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French. - Cell cycle arrest and DNA endoreduplication following p21Waf1/Cip1 expression.
Bates S, Ryan KM, Phillips AC, Vousden KH. Bates S, et al. Oncogene. 1998 Oct 1;17(13):1691-703. doi: 10.1038/sj.onc.1202104. Oncogene. 1998. PMID: 9796698 - G1 arrest and down-regulation of cyclin E/cyclin-dependent kinase 2 by the protein kinase inhibitor staurosporine are dependent on the retinoblastoma protein in the bladder carcinoma cell line 5637.
Schnier JB, Nishi K, Goodrich DW, Bradbury EM. Schnier JB, et al. Proc Natl Acad Sci U S A. 1996 Jun 11;93(12):5941-6. doi: 10.1073/pnas.93.12.5941. Proc Natl Acad Sci U S A. 1996. PMID: 8650198 Free PMC article. - The Interplay between Noncoding RNAs and p21 Signaling in Gastrointestinal Cancer: From Tumorigenesis to Metastasis.
Rahmani F, Zandigohar M, Safavi P, Behzadi M, Ghorbani Z, Payazdan M, Ferns G, Hassanian SM, Avan A. Rahmani F, et al. Curr Pharm Des. 2023;29(10):766-776. doi: 10.2174/1381612829666230306123455. Curr Pharm Des. 2023. PMID: 36876835 Review.
Cited by
- Merkel Cell Polyomavirus Large T Antigen is Dispensable in G2 and M-Phase to Promote Proliferation of Merkel Cell Carcinoma Cells.
Houben R, Ebert M, Hesbacher S, Kervarrec T, Schrama D. Houben R, et al. Viruses. 2020 Oct 14;12(10):1162. doi: 10.3390/v12101162. Viruses. 2020. PMID: 33066686 Free PMC article. - Mechanisms of radiation toxicity in transformed and non-transformed cells.
Panganiban RA, Snow AL, Day RM. Panganiban RA, et al. Int J Mol Sci. 2013 Jul 31;14(8):15931-58. doi: 10.3390/ijms140815931. Int J Mol Sci. 2013. PMID: 23912235 Free PMC article. Review. - The transcription factors TFE3 and TFEB amplify p53 dependent transcriptional programs in response to DNA damage.
Brady OA, Jeong E, Martina JA, Pirooznia M, Tunc I, Puertollano R. Brady OA, et al. Elife. 2018 Dec 6;7:e40856. doi: 10.7554/eLife.40856. Elife. 2018. PMID: 30520728 Free PMC article. - Activation of Raf-1/MEK-1/2/p42/44(MAPK) cascade alone is sufficient to uncouple LDL receptor expression from cell growth.
Kapoor GS, Atkins BA, Mehta KD. Kapoor GS, et al. Mol Cell Biochem. 2002 Jul;236(1-2):13-22. doi: 10.1023/a:1016185928871. Mol Cell Biochem. 2002. PMID: 12190111 - CMTM6 inhibits tumor growth and reverses chemoresistance by preventing ubiquitination of p21 in hepatocellular carcinoma.
Huang Y, Zhu Y, Yang J, Pan Q, Zhao J, Song M, Yang C, Han Y, Tang Y, Wang Q, He J, Li Y, He J, Chen H, Weng D, Xiang T, Xia JC. Huang Y, et al. Cell Death Dis. 2022 Mar 19;13(3):251. doi: 10.1038/s41419-022-04676-1. Cell Death Dis. 2022. PMID: 35304440 Free PMC article.
References
- Beamish H, Williams R, Chen P, Lavin M F. Defect in multiple cell cycle checkpoints in ataxia-telangiectasia postirradiation. J Biol Chem. 1996;271:20486–20493. - PubMed
- Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. - PubMed
- Cardoso M, Leonhardt H, Nadal-Ginard B. Reversal of terminal differentiation and control of DNA replication: cyclin A and Cdk2 specifically localize at subnuclear sites of DNA replication. Cell. 1993;74:979–992. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous