Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis - PubMed (original) (raw)
. 1998 Jan 6;95(1):282-7.
doi: 10.1073/pnas.95.1.282.
E Jacquemin, E Sturm, D Cresteil, P J Bosma, J Aten, J F Deleuze, M Desrochers, M Burdelski, O Bernard, R P Oude Elferink, M Hadchouel
Affiliations
- PMID: 9419367
- PMCID: PMC18201
- DOI: 10.1073/pnas.95.1.282
Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis
J M de Vree et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Class III multidrug resistance (MDR) P-glycoproteins (P-gp), mdr2 in mice and MDR3 in man, mediate the translocation of phosphatidylcholine across the canalicular membrane of the hepatocyte. Mice with a disrupted mdr2 gene completely lack biliary phospholipid excretion and develop progressive liver disease, characterized histologically by portal inflammation, proliferation of the bile duct epithelium, and fibrosis. This disease phenotype is very similar to a subtype of progressive familial intrahepatic cholestasis, hallmarked by a high serum gamma-glutamyltransferase (gamma-GT) activity. We report immunohistochemistry for MDR3 P-gp, reverse transcription-coupled PCR sequence analysis, and genomic DNA analysis of MDR3 from two progressive familial intrahepatic cholestasis patients with high serum gamma-GT. Canalicular staining for MDR3 P-gp was negative in liver tissue of both patients. Reverse transcription-coupled PCR sequencing of the first patient's sequence demonstrated a homozygous 7-bp deletion, starting at codon 132, which results in a frameshift and introduces a stop codon 29 codons downstream. The second patient is homozygous for a nonsense mutation in codon 957 (C --> T) that introduces a stop codon (TGA). Our results demonstrate that mutations in the human MDR3 gene lead to progressive familial intrahepatic cholestasis with high serum gamma-GT. The histopathological picture in these patients is very similar to that in the corresponding mdr2(-/-) mouse, in which mdr2 P-gp deficiency induces complete absence of phospholipid in bile.
Figures
Figure 1
Immunohistochemical staining of control and patient livers. (A–C) Normal human control liver. (D–F) Patient 1. (G–I) Patient 2. (A, D, and G) Immunohistochemical detection of cytokeratin 7 in bile duct epithelium cells. Both patient livers have extensive ductular proliferation (dark staining) and metaplasia of hepatocytes to a ductular phenotype near the portal tract (light staining). (B, E, and H) Immunostaining with C219, a monoclonal antibody against all mammalian P-gps. All livers demonstrate canalicular staining. (C, F, and I) Immunostaining with α-REG1 (a polyclonal antibody against MDR3 P-gp). In contrast to normal human liver (C), both patient livers (F and I) reveal absence of canalicular staining for MDR3 P-gp.
Figure 2
Immunohistochemical detection of MDR3 P-gp in a PFIC patient with normal serum γ-GT activity. Canalicular staining of MDR3 P-gp with α-REG1 demonstrates a normal expression level of this protein. Note the portal fibrosis without ductular proliferation as revealed by hematoxylin staining.
Figure 3
Parts of the MDR3 cDNA sequences of patient 1 (B.K.) (A) and patient 2 (A.B.) (B) encompassing the mutations. Normal sequence is depicted left of the sequence of each patient. The box in A indicates the 7-bp deletion (TATATAC) of patient B.K. starting at codon 132, which results in a frameshift and subsequent stop codon 29 codons downstream. The arrow in B indicates the single base-pair substitution in codon 957 (C → T) in patient A.B. that results in a stop codon (CGA → TGA).
Figure 4
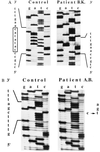
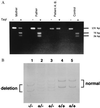
Genomic DNA analysis that confirms the homozygosity of both patients and heterozygosity of their parents. (A) _Taq_I restriction analysis of amplified genomic DNA of patient 2 (A.B.) and his parents. In normal human genomic DNA the _Taq_I digestion of the 131-bp PCR fragment results in two fragments with a length of 75 and 56 bp. In patient 2, the homozygous mutation destroys this restriction site and no fragments are observed. In his heterozygous parents, a combination of the two cleaved fragments and the intact PCR product is observed. (B) PCR–SSCP analysis of genomic DNA, containing the 7-bp mutation, of patient 1 (B.K.) and his family. Denaturated DNA was loaded and electrophoresed in a 15% acrylamide/_N, N_′-methylenebisacrylamide (50:1, wt/wt) gel at 4°C. Patient 1 (lane 1), father (lane 2), mother (lane 3), sister (lane 4), and control (lane 5). In patient 1, only the deleted forms (sense and antisense) are observed. Both parents show the wild-type and the deleted forms, indicating that they are heterozygotes. The patient’s sister is homozygous for the wild-type form.
Similar articles
- Progressive familial intrahepatic cholestasis with high gamma-glutamyltranspeptidase levels in Taiwanese infants: role of MDR3 gene defect?
Chen HL, Chang PS, Hsu HC, Lee JH, Ni YH, Hsu HY, Jeng YM, Chang MH. Chen HL, et al. Pediatr Res. 2001 Jul;50(1):50-5. doi: 10.1203/00006450-200107000-00011. Pediatr Res. 2001. PMID: 11420418 - Heterozygous MDR3 missense mutation associated with intrahepatic cholestasis of pregnancy: evidence for a defect in protein trafficking.
Dixon PH, Weerasekera N, Linton KJ, Donaldson O, Chambers J, Egginton E, Weaver J, Nelson-Piercy C, de Swiet M, Warnes G, Elias E, Higgins CF, Johnston DG, McCarthy MI, Williamson C. Dixon PH, et al. Hum Mol Genet. 2000 May 1;9(8):1209-17. doi: 10.1093/hmg/9.8.1209. Hum Mol Genet. 2000. PMID: 10767346 - Defect of multidrug-resistance 3 gene expression in a subtype of progressive familial intrahepatic cholestasis.
Deleuze JF, Jacquemin E, Dubuisson C, Cresteil D, Dumont M, Erlinger S, Bernard O, Hadchouel M. Deleuze JF, et al. Hepatology. 1996 Apr;23(4):904-8. doi: 10.1002/hep.510230435. Hepatology. 1996. PMID: 8666348 - Genetic cholestasis, causes and consequences for hepatobiliary transport.
Jansen PL, Sturm E. Jansen PL, et al. Liver Int. 2003 Oct;23(5):315-22. doi: 10.1034/j.1478-3231.2003.00856.x. Liver Int. 2003. PMID: 14708891 Review. - Progressive familial intrahepatic cholestasis.
Jacquemin E. Jacquemin E. Clin Res Hepatol Gastroenterol. 2012 Sep;36 Suppl 1:S26-35. doi: 10.1016/S2210-7401(12)70018-9. Clin Res Hepatol Gastroenterol. 2012. PMID: 23141890 Review.
Cited by
- Mutations in DCDC2 (doublecortin domain containing protein 2) in neonatal sclerosing cholangitis.
Grammatikopoulos T, Sambrotta M, Strautnieks S, Foskett P, Knisely AS, Wagner B, Deheragoda M, Starling C, Mieli-Vergani G, Smith J; University of Washington Center for Mendelian Genomics; Bull L, Thompson RJ. Grammatikopoulos T, et al. J Hepatol. 2016 Dec;65(6):1179-1187. doi: 10.1016/j.jhep.2016.07.017. Epub 2016 Jul 25. J Hepatol. 2016. PMID: 27469900 Free PMC article. - Knockout of microRNA-21 reduces biliary hyperplasia and liver fibrosis in cholestatic bile duct ligated mice.
Kennedy LL, Meng F, Venter JK, Zhou T, Karstens WA, Hargrove LA, Wu N, Kyritsi K, Greene J, Invernizzi P, Bernuzzi F, Glaser SS, Francis HL, Alpini G. Kennedy LL, et al. Lab Invest. 2016 Dec;96(12):1256-1267. doi: 10.1038/labinvest.2016.112. Epub 2016 Oct 24. Lab Invest. 2016. PMID: 27775690 Free PMC article. - Hepatobiliary anomalies associated with ABCB4/MDR3 deficiency in adults: a pictorial essay.
Benzimra J, Derhy S, Rosmorduc O, Menu Y, Poupon R, Arrivé L. Benzimra J, et al. Insights Imaging. 2013 Jun;4(3):331-8. doi: 10.1007/s13244-013-0243-y. Epub 2013 Apr 17. Insights Imaging. 2013. PMID: 23591976 Free PMC article. - Cholestasis.
Elferink RO. Elferink RO. Gut. 2003 May;52 Suppl 2(Suppl 2):ii42-8. doi: 10.1136/gut.52.suppl_2.ii42. Gut. 2003. PMID: 12651881 Free PMC article. Review. - ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport.
Klucken J, Büchler C, Orsó E, Kaminski WE, Porsch-Ozcürümez M, Liebisch G, Kapinsky M, Diederich W, Drobnik W, Dean M, Allikmets R, Schmitz G. Klucken J, et al. Proc Natl Acad Sci U S A. 2000 Jan 18;97(2):817-22. doi: 10.1073/pnas.97.2.817. Proc Natl Acad Sci U S A. 2000. PMID: 10639163 Free PMC article.
References
- Keppler D, Arias I M. FASEB J. 1997;11:15–18. - PubMed
- Borst P, Schinkel A H, Smit J J M, Wagenaar E, van Deemter L, Smith A J, Eijdems E W, Baas F, Zaman G J. Pharmacol Ther. 1993;60:289–299. - PubMed
- Gottesman M M, Pastan I. Annu Rev Biochem. 1993;62:385–427. - PubMed
- Smith A J, Timmermans-Hereijgers J L P M, Roelofsen B, Wirtz K W A, Blitterswijk W J, Smit J J M, Schinkel A H, Borst P. FEBS Lett. 1994;354:263–266. - PubMed
- Reutz S, Gros P. Cell. 1994;77:1071–1081. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous