Minimal requirement for a lentivirus vector based on human immunodeficiency virus type 1 - PubMed (original) (raw)
Minimal requirement for a lentivirus vector based on human immunodeficiency virus type 1
V N Kim et al. J Virol. 1998 Jan.
Abstract
The use of human immunodeficiency virus vectors for gene therapy is hampered by concern over their safety. This concern might be ameliorated, in part, if the viral accessory genes and proteins could be eliminated from the vector genomes and particles. Here we describe a minimal vector system that is capable of transducing nondividing cells and which does not contain tat, vif, vpr, vpu, and nef.
Figures
FIG. 1
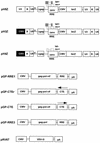
Basic components of the packaging system. Vector genome plasmids pH5Z, pH3Z, and pH4Z were derived from pWI3 (24) and inserted into pBluescript KS+ (Stratagene). They have a number of structural features in common. To achieve efficient packaging by HIV cores, the vectors contain the first 778 nt of gag (36). A frameshift mutation created by filling in at the _Cla_I site (HIV-1 HXB2 coordinate 830 [GenBank accession no. M28248]) prevents translation of these gag sequences. The coordinates for HIV-1 sequences follow the Los Alamos numbering system (32). The remaining gag-pol sequences were removed by a deletion between _Pst_I (HXB2 nt 1415) and _Eco_RI (HXB2 nt 5743). A second deletion between _Nde_I (HXB2 nt 6402) and _Bgl_II (HXB2 nt 7620) removes part of env. The remaining HIV-1 sequences in the vectors include RRE and rev to support efficient mRNA export. The β-galactosidase reporter gene is expressed from an internal HCMV promoter. The differences among the three vector constructs, pH5Z, pH3Z, and pH4Z, are described in the text. HIV-1 gag-pol gene expression plasmids pGP-RRE1, pGP-CTEr, pGP-CTE, and pGP-RRE3 were constructed by first inserting the _Nar_I-_Eco_RI gag-pol fragment (HXB2 nt 637 to 5743) from pWI3 into pCI-neo (Promega). The _Sty_I-_Sty_I fragment containing RRE (HXB2 nt 7721 to 8053) of pWI3 was inserted downstream of the gag-pol coding region, resulting in pGP-RRE1 and pGP-RRE3. In the case of pGP-RRE3, a frameshift mutation in vif was introduced by filling in of the _Nde_I site (HXB2 nt 5122). The CTE (MPMV nt 7886 to 8373 [GenBank accession no. M12349]) was derived from an MPMV proviral clone, pSHRM15 (a kind gift from Eric Hunter), and inserted in either the reverse (pGP-CTEr) or the correct (pGP-CTE) orientation. VSV-G was expressed from the HCMV immediate-early enhancer-promoter in plasmid pRV67 (42a). CMV is the HCMV promoter, Ψ is the HIV-1 packaging signal, lacZ is the β-galactosidase-encoding gene, and pA is the polyadenylation signal. The orientations of the CTE are indicated by arrows as follows: ← for the reverse and → for the correct orientation.
FIG. 2
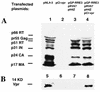
Western blot analysis of viral proteins in viral particles produced by four-plasmid cotransfection. Eight micrograms of pNL4-3, 6 μg of pGP-RRE3, 7 μg of pH4Z, 4 μg of pRV67, and 3 μg of pCI-vpr were transfected, and the total amount of DNA was kept at 20 μg by addition of pCI-neo. At 48 h after transfection, viral pellets were collected from 1 ml of supernatant and separated on sodium dodecyl sulfate–10% (A) or –20% (B) polyacrylamide gels. Expression of viral proteins was visualized by using HIV-1-positive human serum (A) or rabbit anti-Vpr serum (B). Kb, kilodaltons.
Similar articles
- An inducible packaging cell system for safe, efficient lentiviral vector production in the absence of HIV-1 accessory proteins.
Pacchia AL, Adelson ME, Kaul M, Ron Y, Dougherty JP. Pacchia AL, et al. Virology. 2001 Mar 30;282(1):77-86. doi: 10.1006/viro.2000.0787. Virology. 2001. PMID: 11259192 - Development of an Rev-independent, minimal simian immunodeficiency virus-derived vector system.
Pandya S, Boris-Lawrie K, Leung NJ, Akkina R, Planelles V. Pandya S, et al. Hum Gene Ther. 2001 May 1;12(7):847-57. doi: 10.1089/104303401750148847. Hum Gene Ther. 2001. PMID: 11339901 - A third-generation lentivirus vector with a conditional packaging system.
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. Dull T, et al. J Virol. 1998 Nov;72(11):8463-71. doi: 10.1128/JVI.72.11.8463-8471.1998. J Virol. 1998. PMID: 9765382 Free PMC article. - Anti-HIV-1 gene expressing lentiviral vectors as an adjunctive therapy for HIV-1 infection.
Morris KV, Rossi JJ. Morris KV, et al. Curr HIV Res. 2004 Apr;2(2):185-91. doi: 10.2174/1570162043484906. Curr HIV Res. 2004. PMID: 15078182 Review. - Lentiviral vectors for gene therapy of HIV-1 infection.
Mautino MR. Mautino MR. Curr Gene Ther. 2002 Feb;2(1):23-43. doi: 10.2174/1566523023348165. Curr Gene Ther. 2002. PMID: 12108972 Review.
Cited by
- RelA and mitogen-activated protein kinase kinase kinases potently enhance lentiviral vector production.
Yamaoka S. Yamaoka S. Biochem Biophys Rep. 2024 Feb 1;37:101637. doi: 10.1016/j.bbrep.2024.101637. eCollection 2024 Mar. Biochem Biophys Rep. 2024. PMID: 38328371 Free PMC article. - The significance of controlled conditions in lentiviral vector titration and in the use of multiplicity of infection (MOI) for predicting gene transfer events.
Zhang B, Metharom P, Jullie H, Ellem KA, Cleghorn G, West MJ, Wei MQ. Zhang B, et al. Genet Vaccines Ther. 2004 Aug 4;2(1):6. doi: 10.1186/1479-0556-2-6. Genet Vaccines Ther. 2004. PMID: 15291957 Free PMC article. - The Impact of Cellular Proliferation on the HIV-1 Reservoir.
Virgilio MC, Collins KL. Virgilio MC, et al. Viruses. 2020 Jan 21;12(2):127. doi: 10.3390/v12020127. Viruses. 2020. PMID: 31973022 Free PMC article. Review. - Towards a clinically relevant lentiviral transduction protocol for primary human CD34 hematopoietic stem/progenitor cells.
Millington M, Arndt A, Boyd M, Applegate T, Shen S. Millington M, et al. PLoS One. 2009 Jul 30;4(7):e6461. doi: 10.1371/journal.pone.0006461. PLoS One. 2009. PMID: 19649289 Free PMC article. - Development of multigene and regulated lentivirus vectors.
Reiser J, Lai Z, Zhang XY, Brady RO. Reiser J, et al. J Virol. 2000 Nov;74(22):10589-99. doi: 10.1128/jvi.74.22.10589-10599.2000. J Virol. 2000. PMID: 11044103 Free PMC article.
References
- Barillari G, Gendelman R, Gallo R C, Ensoli B. The Tat protein of human immunodeficiency virus type 1, a growth factor for AIDS Kaposi sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. Proc Natl Acad Sci USA. 1993;90:7941–7945. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources