The immediate-early gene product encoded by open reading frame 57 of herpesvirus saimiri modulates gene expression at a posttranscriptional level - PubMed (original) (raw)
The immediate-early gene product encoded by open reading frame 57 of herpesvirus saimiri modulates gene expression at a posttranscriptional level
A Whitehouse et al. J Virol. 1998 Jan.
Abstract
The herpesvirus saimiri (HVS) immediate-early gene product encoded by open reading frame (ORF) 57 shares limited amino acid homology with HSV-1 ICP27 and Epstein-Barr virus BMLF1, both regulatory proteins. The ORF 57 gene has been proposed to be spliced based on the genome sequence, and here we confirm the intron-exon structure of the gene. We also demonstrate that a cDNA construct of the ORF 57 gene product represses the transactivating capability of the ORF 50a gene product (which is produced from a spliced transcript), but activates that of ORF 50b (an unspliced transcript). Further analyses with cotransfection experiments show that ORF 57 can either activate or repress expression from a range of both early and late HVS promoters, depending on the target gene. These results indicate that repression of gene expression mediated by the ORF 57 gene product is dependent on the presence of an intron within the target gene encoding region. Furthermore, Northern blot analysis demonstrates that the levels of mRNA transcribed from genes not containing an intron are not significantly affected in the presence of the ORF 57 gene product. This suggests that it regulates gene expression through a posttranscriptional mechanism.
Figures
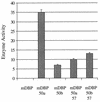
FIG. 1
Response of transactivation capability of ORF 50a and ORF 50b to the ORF 57 gene product. OMK cells were transfected with 2 μg of pAWCAT2 and pAW_Hin_cII or pAW_Pst_I in the absence or presence of pRSVORF57, respectively. Cells were harvested at 48 h posttransfection, and cell extracts were assayed for CAT activity as previously described. Percentages of acetylation were calculated by scintillation counting of the appropriate regions of the chromatography plate and are shown in a graphical format, and the variations between three replicated assays are indicated.
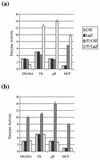
FIG. 2
Response of HVS promoters to the ORF 57 gene product. HVS promoters from the DNA polymerase gene (DNAPol), thymidine kinase gene (TK), major capsid protein gene (MCP), and glycoprotein B (gB) were cloned upstream of the CAT and β-Gal coding regions. (a) The CAT constructs contained the small t antigen intron and SV40 poly(A) region, whereas the β-Gal constructs contained only the SV40 poly(A) signal. OMK cells were transfected with pDPCAT1, pTKCAT1, pMCPCAT1, pgBCAT1, pDPLacZ1, pTKLacZ1, pMCPLacZ1, and pgBLacZ1 in the absence or presence of pRSVORF57. (b) The CAT constructs contained only the SV40 poly(A) region, whereas the β-Gal constructs contained the small t antigen intron and the SV40 poly(A) signal. OMK cells were transfected with pDPCAT2, pTKCAT2, pMCPCAT2, pgBCAT2, pDPLacZ2, pTKLacZ2, pMCPLacZ2, and pgBLacZ2 in the absence or presence of pRSVORF57. Cells were harvested at 48 h posttransfection, and cell extracts were assayed for CAT and β-Gal activity. Percentages of CAT acetylation were calculated by scintillation counting of the appropriate regions of the chromatography plate. The activity of β-Gal was determined by the rate of production of _ortho_-nitrophenol from the substrate _ortho_-nitrophenyl galactoside. Results are shown in graphical format, and the variations between three replicated assays are indicated.
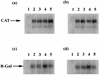
FIG. 3
Northern blot analysis of HVS promoter CAT and β-Gal constructs in response to the ORF 57 gene product. RNA was isolated from OMK cells transfected with the constructs pRSVORF57 (lane 1), pTKCAT1 (lane 2), pTKCAT2 (lane 3), pTKCAT1 and pRSVORF57 (lane 4), or pTKCAT2 and pRSVORF57 (lane 5) (a) plus pgBCAT (b), pTKLacZ (c), and pgBLacZ (d), separated by electrophoresis on a 1% denaturing formaldehyde agarose gel, blotted onto a nylon membrane, and hybridized with radiolabelled probes specific for the CAT and β-Gal coding regions.
Similar articles
- The open reading frame 57 gene product of herpesvirus saimiri shuttles between the nucleus and cytoplasm and is involved in viral RNA nuclear export.
Goodwin DJ, Hall KT, Stevenson AJ, Markham AF, Whitehouse A. Goodwin DJ, et al. J Virol. 1999 Dec;73(12):10519-24. doi: 10.1128/JVI.73.12.10519-10524.1999. J Virol. 1999. PMID: 10559371 Free PMC article. - The open reading frame (ORF) 50a gene product regulates ORF 57 gene expression in herpesvirus saimiri.
Whitehouse A, Cooper M, Hall KT, Meredith DM. Whitehouse A, et al. J Virol. 1998 Mar;72(3):1967-73. doi: 10.1128/JVI.72.3.1967-1973.1998. J Virol. 1998. PMID: 9499050 Free PMC article. - The Herpesvirus saimiri replication and transcription activator acts synergistically with CCAAT enhancer binding protein alpha to activate the DNA polymerase promoter.
Wakenshaw L, Walters MS, Whitehouse A. Wakenshaw L, et al. J Virol. 2005 Nov;79(21):13548-60. doi: 10.1128/JVI.79.21.13548-13560.2005. J Virol. 2005. PMID: 16227275 Free PMC article. - Bovine herpesvirus 4: genomic organization and relationship with two other gammaherpesviruses, Epstein-Barr virus and herpesvirus saimiri.
Lomonte P, Bublot M, van Santen V, Keil G, Pastoret PP, Thiry E. Lomonte P, et al. Vet Microbiol. 1996 Nov;53(1-2):79-89. doi: 10.1016/s0378-1135(96)01236-9. Vet Microbiol. 1996. PMID: 9011000 Review. - gamma-2 Herpes virus post-transcriptional gene regulation.
Boyne JR, Whitehouse A. Boyne JR, et al. Clin Microbiol Infect. 2006 Feb;12(2):110-7. doi: 10.1111/j.1469-0691.2005.01317.x. Clin Microbiol Infect. 2006. PMID: 16441447 Review.
Cited by
- The human herpesvirus 8 homolog of Epstein-Barr virus SM protein (KS-SM) is a posttranscriptional activator of gene expression.
Gupta AK, Ruvolo V, Patterson C, Swaminathan S. Gupta AK, et al. J Virol. 2000 Jan;74(2):1038-44. doi: 10.1128/jvi.74.2.1038-1044.2000. J Virol. 2000. PMID: 10623771 Free PMC article. - Kaposi's sarcoma-associated herpesvirus open reading frame 57 encodes a posttranscriptional regulator with multiple distinct activities.
Kirshner JR, Lukac DM, Chang J, Ganem D. Kirshner JR, et al. J Virol. 2000 Apr;74(8):3586-97. doi: 10.1128/jvi.74.8.3586-3597.2000. J Virol. 2000. PMID: 10729134 Free PMC article. - Herpesvirus saimiri open reading frame 50 (Rta) protein reactivates the lytic replication cycle in a persistently infected A549 cell line.
Goodwin DJ, Walters MS, Smith PG, Thurau M, Fickenscher H, Whitehouse A. Goodwin DJ, et al. J Virol. 2001 Apr;75(8):4008-13. doi: 10.1128/JVI.75.8.4008-4013.2001. J Virol. 2001. PMID: 11264393 Free PMC article. - The open reading frame 57 gene product of herpesvirus saimiri shuttles between the nucleus and cytoplasm and is involved in viral RNA nuclear export.
Goodwin DJ, Hall KT, Stevenson AJ, Markham AF, Whitehouse A. Goodwin DJ, et al. J Virol. 1999 Dec;73(12):10519-24. doi: 10.1128/JVI.73.12.10519-10524.1999. J Virol. 1999. PMID: 10559371 Free PMC article. - Kaposi's sarcoma-associated herpesvirus encodes a bZIP protein with homology to BZLF1 of Epstein-Barr virus.
Lin SF, Robinson DR, Miller G, Kung HJ. Lin SF, et al. J Virol. 1999 Mar;73(3):1909-17. doi: 10.1128/JVI.73.3.1909-1917.1999. J Virol. 1999. PMID: 9971770 Free PMC article.
References
- Bublot M, Manet E, Lequarre A S, Albrecht J C, Nicholas J, Fleckenstein B, Pastoret P P, Thiry E. Genetic relationships between bovine herpesvirus 4 and the gamma-herpesviruses Epstein-Barr and herpesvirus saimiri. Virology. 1992;190:654–665. - PubMed
- Everett R D. The products of herpes simplex virus type 1 (HSV-1) immediate early genes 1, 2 and 3 can activate HSV-1 gene expression in trans. J Gen Virol. 1986;76:2507–2513. - PubMed
- Fleckenstein B, Desrosiers R C. Herpesvirus saimiri and herpesvirus ateles. In: Roizman B, editor. The herpesviruses. Vol. 1. New York, N.Y: Plenum Press; 1982. pp. 253–332.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources