Hoxc13 mutant mice lack external hair - PubMed (original) (raw)
Hoxc13 mutant mice lack external hair
A R Godwin et al. Genes Dev. 1998.
Abstract
Hox genes are usually expressed temporally and spatially in a colinear manner with respect to their positions in the Hox complex. Consistent with the expected pattern for a paralogous group 13 member, early embryonic Hoxc13 expression is found in the nails and tail. Hoxc13 is also expressed in vibrissae, in the filiform papillae of the tongue, and in hair follicles throughout the body; a pattern that apparently violates spatial colinearity. Mice carrying mutant alleles of Hoxc13 have been generated by gene targeting. Homozygotes have defects in every region in which gene expression is seen. The most striking defect is brittle hair resulting in alopecia (hairless mice). One explanation for this novel role is that Hoxc13 has been recruited for a function common to hair, nail, and filiform papilla development.
Figures
Figure 1
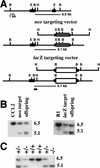
Targeting vectors and Southern blot analysis. (A) Genomic structure and targeting vectors. Large solid boxes represent Hoxc13 exons, dark gray boxes the Pol II–neo cassette, the shaded boxes the lacZ MC1 neo cassette, (B) _Bam_HI, (E) _Eco_RI, (H) _Hin_dIII, and (X) _Xho_I. The position of the 5′-flanking probe used for Southern transfer analysis is indicated by the small solid box. The first line shows the wild-type genomic structure and subsequent lines show the Hoxc13neo and Hoxc13lacZ targeting vectors and the genomic structure resulting from homologous recombination. (B) Southern blot analysis of targeted cell lines and offspring from chimera generated from the targeted ES cell lines. The first three lanes show _Bam_HI–_Xho_I digests of the parental ES cell line CC1.2, a targeted ES cell line, and an offspring from a chimeric male. The second set of three lanes shows a _Bam_HI digest of DNA from the parental ES cell line R1, a targeted ES cell line, and an offspring from a chimeric male. (C) Genotype of a litter from a Hoxc-13neo heterozygote intercross. Southern transfer analysis of _Bam_HI–_Xho_I-digested DNA was performed and shows two wild-type mice carrying only the 6.5-kb fragment, three heterozygous animals with both the 6.5- and 5.1-kb fragments, and one mutant homozygote with only the 5.1-kb fragment.
Figure 2
Hoxc13 gene expression. (A–C,E–H) β-Gal staining of heterozygous Hoxc13lacZ embryos at the noted embryonic ages. D shows RNA in situ hybridization of a Swiss Webster embryo at E13.5. (A) E10.5 embryo. Tail expression is indicated by arrows. (B) E12.5 embryo. Black arrows show limb expression in wrist and ankle regions and white arrow shows tail expression. (C) E13.5 embryo. White arrow shows vibrissae expression; the black arrow shows tail expression; white arrowhead shows expression in a thin layer of the digits. (D) E13.5 embryo. White arrow shows vibrissae expression; black arrow shows tail expression; the black arrowhead shows faint digit expression. (E) E15.5 embryo. White arrow shows continuing vibrissae staining; black arrow shows beginning hair follicle expression. (F) E16.5 embryo. The white arrow shows lack of surface vibrissae expression; the black arrow shows hair follicle expression. (G) E17.5 embryo. The black arrow highlights hair follicle expression first seen at E15.5; the white arrow indicates expression in the second set of hair follicles. The black arrowhead indicates expression in the cilia. (H) E17.5 embryo. The white arrow indicates strong dorsal nail β-gal staining; the black arrow points to fainter ventral staining.
Figure 3
Overt Hoxc13 phenotypes. (A) Hoxc13neo wild-type non-Agouti mouse. (B) Hoxc13neo heterozygous Agouti mouse. (C) Hoxc13neo homozygote. (D) Close-up of Hoxc13neo homozygote head. (E) Close-up of Hoxc13neo homozygote face. The black arrow shows lack of protruding vibrissae; the black arrowhead shows hair remnants under skin surface. (F) Close-up of Hoxc13neo homozygote lower limb. The black arrow shows rare protruding hair. (G) Wild-type nails. (H) Nails from a Hoxc13neo homozygote.
Figure 4
Hoxc13 expression in the hair and histology. (A) β-Gal staining of postnatal day (P) 7 skin of Hoxc13lacZ heterozygote. (B) β-Gal staining (blue) and anti-K2.6 immunohistochemistry (brown) of Hoxc13lacZ homozygous skin. The black arrow shows expression in rapidly dividing keratinocytes; the black arrowhead shows expression in single nuclei in companion layer. (C) β-Gal staining and anti-K2.6 immunohistochemistry of Hoxc13lacZ homozygous skin. Black arrowheads indicate nuclear staining in nuclei of the companion layer. Scale bar, 20 μm (shows magnification for B and C).
Figure 5
Tongue expression and histology. (A,E) β-Gal staining of P6 tongues from a Hoxc13lacZ heterozygote and homozygote, respectively. (B–D) β-Gal staining (blue) and anti-K2.6 immunohistochemistry (brown) on P6 tongues, whereas F–H show β-gal staining and hematoxylin–eosin staining. H&E staining was performed as described previously (Mansour et al. 1993). Scale bar, (H), 50 μm (shows the magnification for B–D and F–H). B and F are of wild-type sections, C and G from a Hoxc13lacZ heterozygote, and D and H from a Hoxc13lacZ homozygote. Arrows in C and G indicate β-gal staining in the base of the spines. Arrows in D and H show β-gal staining extending into the malformed spines.
Figure 6
Scanning electron micrographs of adult tongues. Scale bar in A is 50 μm (A–C). (A) Wild-type tongue. (B) Hoxc13lacZ heterozygote tongue. (C) Hoxc13lacZ homozygote tongue. The small white arrowhead shows a fungiform papilla; the large white arrowhead shows a filiform papilla remnant; the white arrow shows a broken-off filiform papilla piece on the tongue surface.
Figure 6
Scanning electron micrographs of adult tongues. Scale bar in A is 50 μm (A–C). (A) Wild-type tongue. (B) Hoxc13lacZ heterozygote tongue. (C) Hoxc13lacZ homozygote tongue. The small white arrowhead shows a fungiform papilla; the large white arrowhead shows a filiform papilla remnant; the white arrow shows a broken-off filiform papilla piece on the tongue surface.
Figure 6
Scanning electron micrographs of adult tongues. Scale bar in A is 50 μm (A–C). (A) Wild-type tongue. (B) Hoxc13lacZ heterozygote tongue. (C) Hoxc13lacZ homozygote tongue. The small white arrowhead shows a fungiform papilla; the large white arrowhead shows a filiform papilla remnant; the white arrow shows a broken-off filiform papilla piece on the tongue surface.
Figure 7
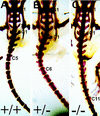
Axial skeleton phenotype. (A) Wild-type skeleton. (B) Hoxc-13neo heterozygote skeleton. (C) Hoxc-13neo homozygote skeleton. (S1) represents sacral vertebra 1, (S4) sacral vertebra 4, and (C1) caudal vertebra 1. In addition, the last caudal vertebra with a lateral projection is labeled for each genotype (i.e., caudal vertebra C5 in A). Preparation of skeletons was as described previously (Davis and Capecchi 1994).
Similar articles
- Hox in hair growth and development.
Awgulewitsch A. Awgulewitsch A. Naturwissenschaften. 2003 May;90(5):193-211. doi: 10.1007/s00114-003-0417-4. Epub 2003 Apr 26. Naturwissenschaften. 2003. PMID: 12743702 Review. - The nude mutant gene Foxn1 is a HOXC13 regulatory target during hair follicle and nail differentiation.
Potter CS, Pruett ND, Kern MJ, Baybo MA, Godwin AR, Potter KA, Peterson RL, Sundberg JP, Awgulewitsch A. Potter CS, et al. J Invest Dermatol. 2011 Apr;131(4):828-37. doi: 10.1038/jid.2010.391. Epub 2010 Dec 30. J Invest Dermatol. 2011. PMID: 21191399 Free PMC article. - Hair defects in Hoxc13 mutant mice.
Godwin AR, Capecchi MR. Godwin AR, et al. J Investig Dermatol Symp Proc. 1999 Dec;4(3):244-7. doi: 10.1038/sj.jidsp.5640221. J Investig Dermatol Symp Proc. 1999. PMID: 10674376 Review. - Homeotic transformations and limb defects in Hox A11 mutant mice.
Small KM, Potter SS. Small KM, et al. Genes Dev. 1993 Dec;7(12A):2318-28. doi: 10.1101/gad.7.12a.2318. Genes Dev. 1993. PMID: 7902826 - Naked (N) mutant mice carry a nonsense mutation in the homeobox of Hoxc13.
Perez CJ, Mecklenburg L, Fernandez A, Cantero M, de Souza TA, Lin K, Dent SYR, Montoliu L, Awgulewitsch A, Benavides F. Perez CJ, et al. Exp Dermatol. 2022 Mar;31(3):330-340. doi: 10.1111/exd.14469. Epub 2021 Oct 25. Exp Dermatol. 2022. PMID: 34657330 Free PMC article.
Cited by
- Adaptive evolution of the Hox gene family for development in bats and dolphins.
Liang L, Shen YY, Pan XW, Zhou TC, Yang C, Irwin DM, Zhang YP. Liang L, et al. PLoS One. 2013 Jun 25;8(6):e65944. doi: 10.1371/journal.pone.0065944. Print 2013. PLoS One. 2013. PMID: 23825528 Free PMC article. - HOXC10 is significantly overexpressed in colorectal cancer.
Enteghami M, Ghorbani M, Zamani M, Galehdari H. Enteghami M, et al. Biomed Rep. 2020 Sep;13(3):18. doi: 10.3892/br.2020.1325. Epub 2020 Jul 28. Biomed Rep. 2020. PMID: 32765857 Free PMC article. - Large scale transgenic and cluster deletion analysis of the HoxD complex separate an ancestral regulatory module from evolutionary innovations.
Spitz F, Gonzalez F, Peichel C, Vogt TF, Duboule D, Zákány J. Spitz F, et al. Genes Dev. 2001 Sep 1;15(17):2209-14. doi: 10.1101/gad.205701. Genes Dev. 2001. PMID: 11544178 Free PMC article. - The mammary bud as a skin appendage: unique and shared aspects of development.
Mikkola ML, Millar SE. Mikkola ML, et al. J Mammary Gland Biol Neoplasia. 2006 Oct;11(3-4):187-203. doi: 10.1007/s10911-006-9029-x. J Mammary Gland Biol Neoplasia. 2006. PMID: 17111222 Review. - Control of Hoxd gene transcription in the mammary bud by hijacking a preexisting regulatory landscape.
Schep R, Necsulea A, Rodríguez-Carballo E, Guerreiro I, Andrey G, Nguyen Huynh TH, Marcet V, Zákány J, Duboule D, Beccari L. Schep R, et al. Proc Natl Acad Sci U S A. 2016 Nov 29;113(48):E7720-E7729. doi: 10.1073/pnas.1617141113. Epub 2016 Nov 15. Proc Natl Acad Sci U S A. 2016. PMID: 27856734 Free PMC article.
References
- Akam M. Hox and HOM: Homologous gene clusters in insects and vertebrates. Cell. 1989;57:347–349. - PubMed
- Bieberich CJ, Ruddle FH, Stenn KS. Differential expression of the Hox 3.1 gene in adult mouse skin. Ann NY Acad Sci. 1991;642:346–353. - PubMed
- Boulet AM, Capecchi MR. Targeted disruption of hoxc-4 causes esophageal defects and vertebral transformations. Dev Biol. 1996;177:232–249. - PubMed
- Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases