Functional separation of pseudopod extension and particle internalization during Fc gamma receptor-mediated phagocytosis - PubMed (original) (raw)
Comparative Study
Functional separation of pseudopod extension and particle internalization during Fc gamma receptor-mediated phagocytosis
M B Lowry et al. J Exp Med. 1998.
Abstract
Receptors for the Fc portion of immunoglobulin (Ig)G (Fc gamma R) mediate phagocytosis of IgG-opsonized particles by a process that can be divided into four major steps: receptor-ligand binding, pseudopod extension, internalization, and lysosomal fusion. We have expressed single classes of Fc gamma R in COS fibroblasts to examine the structural determinants necessary to complete the four steps of phagocytosis. Using phase contrast, fluorescence, confocal, and electron microscopy we have demonstrated that Fc gamma R-expressing COS cells can phagocytose in a manner similar to that of professional phagocytes. We have further analyzed the capacity of the three classes of Fc gamma R to phagocytose, placing special emphasis on the Fc gamma RIA-gamma chain complex, which allowed us to examine independently the roles of the ligand-binding unit (Fc gamma RIA) and the signaling unit (gamma chain). We found that receptor complexes containing a conserved tyrosine activation motif (ITAM), as found in the cytoplasmic domain of Fc gamma RIIA and in the gamma chain associated with Fc gamma RIA and Fc gamma RIIIA, readily internalized target particles. In contrast, Fc gamma RIA alone, having no ITAM, was unable to internalize target particles efficiently, but did mediate pseudopod extension. Cotransfection of gamma chain with Fc gamma RIA restored the ability of the receptor to internalize target particles. A mutant Fc gamma RIA in which the cytoplasmic domain had been deleted was also capable of mediating pseudopod extension, showing that neither the gamma chain nor the cytoplasmic domain of Fc gamma RIA were required for this step. Cytochalasin D, an inhibitor of actin polymerization, blocked particle internalization by all Fc gamma R, but did not block pseudopod extension. Staining the Fc gamma RIA COS cells for F-actin and for tyrosine phosphoproteins, we found that actin did not polymerize during Fc gamma RIA-mediated pseudopod extension, nor were tyrosine kinases activated. Our data suggest that pseudopod extension and internalization are functionally distinct steps mediated through different pathways.
Figures
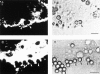
Figure 1
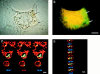
Analysis of FcγR-mediated phagocytosis by phase contrast and confocal microscopy. FcγR-transfected COS cells were incubated with FITC-labeled sheep RBCs opsonized with IgG at 37°C for 1 h to allow internalization to occur. The cells were then subjected to a brief hypotonic lysis to remove externally bound RBCs and were fixed and stained to mark the COS plasma membrane. A and B illustrate a single FcγRIIA-expressing cell stained orange with ethidium bromide examined by both phase contrast and fluorescence microscopy that has internalized several RBC targets (arrows) into large vesicles. In C and D, confocal sections in two axes of the same cells show green RBC targets that appear within the COS membrane labeled with a WGA–rhodamine conjugate appearing red. C shows several sections in the z-axis, whereas D shows the y-axis of the same cell. The third axis, x, is similar to D and is not shown. Bar, 10 μm.
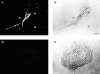
Figure 2
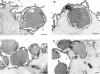
FcγR-transfected COS cells internalize targets into sealed phagosomes that subsequently fuse with lysosomes. Transfected COS cells were incubated with IgG-coated latex beads at 37°C to allow phagocytosis to occur. The cells were then fixed and processed for electron microscopy. In A and B, the plasma membrane was labeled with a WGA–HRP conjugate and reacted with a DAB peroxide solution resulting in an electron-dense band at the membrane surface. A illustrates target beads that have been bound but not sealed within the cell as indicated by the presence of the external membrane label around the beads (arrows). B shows a target bead that has been internalized within a sealed phagosome showing no membrane label, whereas the plasma membrane is densely stained. In C and D, the cells were processed to detect acid phosphatase activity, a lysosomal marker. C shows a bead target that has not been sealed within the cell and does not localize with acid phosphatase activity. D illustrates a bead target that has been internalized and does colocalize with the electron-dense reaction product of acid phosphatase (arrowheads), indicating lysosomal fusion. Bar, 1 μm.
Figure 3
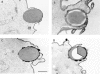
THP-1 monocytes and COS transfectants extend morphologically similar pseudopods. THP-1 monocytes (A and B) and FcγRIA COS transfectants (C and D) were incubated with IgG-opsonized sheep RBCs at 37°C for 45 min (A and C) or 1 h (B and D). The cells were then fixed, labeled with a WGA–HRP conjugate, and processed for electron microscopy as described in the legend to Fig. 2, A and B. In A, a THP-1 monocyte has extended fine pseudopods around an RBC target in an early phase of the phagocytic process. B shows a later phase of pseudopod extension in which the THP-1 cell has drawn the RBC target almost completely into the cell. In C, an FcγRIA COS transfectant has extended fine pseudopods around the RBC targets in an early phase of the process, whereas D shows a later terminal phase in which the RBC is surrounded by the cell, but not sealed within a phagosome. Bar, 1 μm.
Figure 4
Electron micrographs of FcγRIA (A–C) and FcγRIA–γ chain (D) COS transfectants engaged in the cupping phase of phagocytosis. Cells were incubated with or without cytochalasin D for 1 h at 37°C and were then processed for electron microscopy. The cupping phase is observed in the absence (A and B) and presence (C and D) of cytochalasin D. Variations in morphology of the pseudopods were common, as illustrated in A and B in which both examples were not treated with cytochalasin D. D illustrates the method used to measure the extent of pseudopod formation. The circumference of the bead covered by pseudopods is measured in degrees, indicated by the inset white area. Bar, 1 μm.
Figure 5
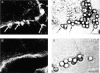
Confocal analysis of F-actin after IgG-coated particle binding to FcγR COS transfectants. Transfected COS cells were incubated with IgG-coated beads for 15 min at 37°C and were then fixed, permeabilized, and stained for F-actin using FITC-phalloidin. A and C show 0.5-μm–thick single confocal sections of F-actin staining, whereas B and D are companion nonconfocal transmitted light phase contrast sections. In A, an FcγRIIA transfectant shows F-actin extensions surrounding bound beads, whereas the parallel B provides the position of the beads. In C, an FcγRIA transfectant does not show an F-actin extensions or increases in F-actin due to target binding above background levels, whereas the parallel D shows the location of the beads. Cells were examined in all planes of focus to detect F-actin extensions as quantified in Table 4. Arrows, F-actin extensions in A, and associated beads in B. Bar, 5 μm.
Figure 6
Analysis of pseudopod extension by confocal microscopy. Parallel samples of transfected COS cells from the experiment described in Fig. 5 were incubated with IgG-coated beads for 15 min at 37°C and were then fixed and stained with a DiI derivative to label the membranes of the cells. A and C show single confocal sections of DiI lipid staining, whereas B and D show companion nonconfocal phase contrast sections. In A and B, an FcγRIIA-transfected cell shows two beads with associated pseudopod extensions in the process of closure, whereas in C and D an FcγRIA-transfected cell shows several terminal pseudopod extensions around bound beads that do not fuse. Arrowheads, beads with associated pseudopod extensions. Bar, 5 μm.
Figure 7
Antiphosphotyrosine staining of FcγR-transfected COS cells after IgG-opsonized particle binding. Transfected COS cells were incubated with IgG-opsonized RBCs for 10 min at 37°C and were then fixed and stained with antiphosphotyrosine mAb 4G10 followed by a secondary FITC-conjugated donkey anti–mouse IgG for detection. A and C show anti-phosphotyrosine staining, whereas B and D show companion nonconfocal phase contrast sections. In A, an FcγRIIA-expressing cell displays tyrosine phosphoprotein accumulation at sites of IgG-opsonized particle binding, indicating triggered tyrosine kinase activity. In B, several cells not expressing FcγR (n, nuclei) can be seen that show only background staining for phosphotyrosine in A, confirming specificity. C and D show an FcγRIA-expressing cell that has bound many IgG-opsonized RBCs, but does not show any phosphotyrosine staining above background levels. Bar, 10 μm.
Similar articles
- Two distinct regions of FC gamma RI initiate separate signalling pathways involved in endocytosis and phagocytosis.
Davis W, Harrison PT, Hutchinson MJ, Allen JM. Davis W, et al. EMBO J. 1995 Feb 1;14(3):432-41. doi: 10.1002/j.1460-2075.1995.tb07019.x. EMBO J. 1995. PMID: 7859733 Free PMC article. - Binding of IgG-opsonized particles to Fc gamma R is an active stage of phagocytosis that involves receptor clustering and phosphorylation.
Sobota A, Strzelecka-Kiliszek A, Gładkowska E, Yoshida K, Mrozińska K, Kwiatkowska K. Sobota A, et al. J Immunol. 2005 Oct 1;175(7):4450-7. doi: 10.4049/jimmunol.175.7.4450. J Immunol. 2005. PMID: 16177087 - Fc gamma receptors differ in their structural requirements for interaction with the tyrosine kinase Syk in the initial steps of signaling for phagocytosis.
Kim MK, Pan XQ, Huang ZY, Hunter S, Hwang PH, Indik ZK, Schreiber AD. Kim MK, et al. Clin Immunol. 2001 Jan;98(1):125-32. doi: 10.1006/clim.2000.4955. Clin Immunol. 2001. PMID: 11141335 - The molecular dissection of Fc gamma receptor mediated phagocytosis.
Indik ZK, Park JG, Hunter S, Schreiber AD. Indik ZK, et al. Blood. 1995 Dec 15;86(12):4389-99. Blood. 1995. PMID: 8541526 Review. - Structure/function relationships of Fc gamma receptors in phagocytosis.
Indik ZK, Park JG, Hunter S, Schreiber AD. Indik ZK, et al. Semin Immunol. 1995 Feb;7(1):45-54. doi: 10.1016/1044-5323(95)90007-1. Semin Immunol. 1995. PMID: 7612895 Review.
Cited by
- Functions of the Fc receptors for immunoglobulin G.
Flesch BK, Neppert J. Flesch BK, et al. J Clin Lab Anal. 2000;14(4):141-56. doi: 10.1002/1098-2825(2000)14:4<141::aid-jcla3>3.0.co;2-0. J Clin Lab Anal. 2000. PMID: 10906767 Free PMC article. Review. No abstract available. - Self inhibition of phagocytosis: the affinity of 'marker of self' CD47 for SIRPalpha dictates potency of inhibition but only at low expression levels.
Tsai RK, Rodriguez PL, Discher DE. Tsai RK, et al. Blood Cells Mol Dis. 2010 Jun 15;45(1):67-74. doi: 10.1016/j.bcmd.2010.02.016. Epub 2010 Mar 17. Blood Cells Mol Dis. 2010. PMID: 20299253 Free PMC article. - Inhibition of "self" engulfment through deactivation of myosin-II at the phagocytic synapse between human cells.
Tsai RK, Discher DE. Tsai RK, et al. J Cell Biol. 2008 Mar 10;180(5):989-1003. doi: 10.1083/jcb.200708043. J Cell Biol. 2008. PMID: 18332220 Free PMC article. - Cross-linking of IgGs bound on circulating neutrophils leads to an activation of endothelial cells: possible role of rheumatoid factors in rheumatoid arthritis-associated vascular dysfunction.
Rollet-Labelle E, Vaillancourt M, Marois L, Newkirk MM, Poubelle PE, Naccache PH. Rollet-Labelle E, et al. J Inflamm (Lond). 2013 Jul 31;10(1):27. doi: 10.1186/1476-9255-10-27. J Inflamm (Lond). 2013. PMID: 23902799 Free PMC article. - Concurrent binding to multiple ligands: kinetic rates of CD16b for membrane-bound IgG1 and IgG2.
Williams TE, Selvaraj P, Zhu C. Williams TE, et al. Biophys J. 2000 Oct;79(4):1858-66. doi: 10.1016/S0006-3495(00)76435-6. Biophys J. 2000. PMID: 11023891 Free PMC article.
References
- Ghazizadeh S, Bolen JB, Fleit HB. Physical and functional association of src-related protein tyrosine kinases with FcγRII in monocytic THP-1 cells. J Biol Chem. 1994;269:8878–8884. - PubMed