Invariant chain-independent function of H-2M in the formation of endogenous peptide-major histocompatibility complex class II complexes in vivo - PubMed (original) (raw)
Invariant chain-independent function of H-2M in the formation of endogenous peptide-major histocompatibility complex class II complexes in vivo
S Kovats et al. J Exp Med. 1998.
Abstract
Efficient loading of major histocompatibility complex class II molecules with peptides requires the invariant chain (Ii) and the class II-like molecule H-2M. Recent in vitro biochemical studies suggest that H2-M may function as a chaperone to rescue empty class II dimers. To test this hypothesis in vivo, we generated mice lacking both Ii and H-2M (Ii-/-M-/-). Antigen presenting cells (APCs) from Ii-/-M-/- mice, as compared with APCs from Ii-/- mice, exhibit a significant reduction in their ability to present self-peptides to a panel of class II I-Ab-restricted T cells. As a consequence of this defect in the loading of self peptides, CD4(+) thymocyte development is profoundly impaired in Ii-/-M-/- mice, resulting in a peripheral CD4(+) T cell population with low levels of T cell receptor expression. These findings are consistent with the idea that H-2M functions as a chaperone in the peptide loading of class II molecules in vivo.
Figures
Figure 1
The absence of Ii chain and H-2M results in synergistic reduction of CD4+ T cell development. Thymocytes from (A) wild-type B6x129 and mutant (B) Ii−/−, (C) Ii−/−M−/−, (D) M−/−, and (E) MHC-II−/− mice were analyzed for expression of CD4, CD8, and TCR-α/β using flow cytometry. Shown are two parameter dot plots of CD8 versus TCR-α/β, in which three electronic gates are indicated, delineating the following populations: CD4−CD8hiTCRhi (box 1), CD4+CD8−TCRhi (box 2), and CD4+CD8loTCRlo (box 3). Also shown are the percentages of cells that fall within each box. Cells in boxes 2 and 3 are >97% CD4+. Splenocytes from wild-type and mutant mice were analyzed for expression of CD4, CD8, and TCR-α/β using flow cytometry. Shown are comparisons of TCR expression on (F) CD4+ T cells and (G) CD8+ T cells in the mice of the five genotypes, as indicated. Data are representative of analyses of four mice of each genotype.
Figure 1
The absence of Ii chain and H-2M results in synergistic reduction of CD4+ T cell development. Thymocytes from (A) wild-type B6x129 and mutant (B) Ii−/−, (C) Ii−/−M−/−, (D) M−/−, and (E) MHC-II−/− mice were analyzed for expression of CD4, CD8, and TCR-α/β using flow cytometry. Shown are two parameter dot plots of CD8 versus TCR-α/β, in which three electronic gates are indicated, delineating the following populations: CD4−CD8hiTCRhi (box 1), CD4+CD8−TCRhi (box 2), and CD4+CD8loTCRlo (box 3). Also shown are the percentages of cells that fall within each box. Cells in boxes 2 and 3 are >97% CD4+. Splenocytes from wild-type and mutant mice were analyzed for expression of CD4, CD8, and TCR-α/β using flow cytometry. Shown are comparisons of TCR expression on (F) CD4+ T cells and (G) CD8+ T cells in the mice of the five genotypes, as indicated. Data are representative of analyses of four mice of each genotype.
Figure 2
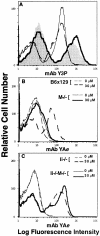
Occupancy of surface class II molecules with high affinity endogenous peptides is diminished in Ii−/−M−/− splenocytes. (A) Cell surface class II expression on splenocytes from wild-type and mutant mice was assessed by the binding of biotinylated mAb Y3P and streptavidin-PE, followed by flow cytometry. Shown are profiles from wild-type B6x129 (shaded histogram), M−/− (thick solid line), Ii−/− (thin dotted line), and Ii−/− M−/− (thin solid line) mice. For assessment of peptide occupancy, splenocytes were incubated in the absence (0 μM) or presence (30 μM) of synthetic Eα52–68 peptide (as indicated) for 3 h at 37°C, before assessment of the resulting Eα52–68–I-Ab complex formation using biotinylated mAb YA_e_ and streptavidin-PE in flow cytometric analyses. Shown is binding of mAb YA_e_ to (B) wild-type B6x129 and M−/− cells and (C) Ii−/− and Ii−/− M−/− cells. Data are representative of four independent experiments.
Figure 3
Endogenous peptides bind with high affinity to purified I-Ab molecules in vitro. Purified I-Ab molecules were incubated with titrated amounts of unlabeled test peptides and a biotinylated indicator peptide Eα52–68. Subsequently, I-Ab molecules were captured on plates, and the amount of biotinylated Eα peptide bound was quantified using Eu3+-streptavidin and fluorometry. Data were normalized to the maximal binding of biotinylated Eα52–68 (65,000–150,000 fluorescence units) in the absence of inhibitor, and are reported as percentage maximal binding of this indicator peptide. Shown are binding curves in the presence of unlabeled Eα52–68 and a peptide that does not bind to I-Ab, L32 2–21. Background signal in the assay was 2,000–5,000 fluorescence units.
Figure 4
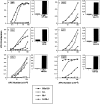
Presentation of select endogenous self peptides requires H-2M even in the absence of Ii-derived CLIP. Activation of antigen-specific T cell hybrids by ex vivo splenic APCs isolated from wild-type and mutant mice. Specificities of the T cell hybrids are as follows: (A) IgM 377–392, (B) β2m 48– 58, (C) LDLr 486–501, (D) actin 163–177, (E) AAT 394–410, and (F) Clp36 138–153. Shown in the left panels (line graphs, A–F) are responses of T cell hybrids to variable numbers of splenocytes in the absence of exogenous peptide; splenocytes derived from wild-type B6x129 (squares), Ii−/− (open circles), M−/− (triangles), and Ii−/−M−/− (closed circles) mice are distinguished as indicated. Shown in the right panels (bar graphs, A–F) are responses of T cell hybrids to M−/− splenocytes in the absence or presence of exogenously supplied cognate peptide (10 μg/ml). IL-2 production by the T cell hybrids was assessed by proliferation of HT-2 cells using an Alamar blue colorimetric assay; results (average of duplicate wells) are expressed as arbitrary units of OD at 570 versus 600 nm. Data are representative of four independent experiments.
Similar articles
- Effect of decreasing the affinity of the class II-associated invariant chain peptide on the MHC class II peptide repertoire in the presence or absence of H-2M.
Honey K, Forbush K, Jensen PE, Rudensky AY. Honey K, et al. J Immunol. 2004 Apr 1;172(7):4142-50. doi: 10.4049/jimmunol.172.7.4142. J Immunol. 2004. PMID: 15034026 - Distinct peptide loading pathways for MHC class II molecules associated with alternative Ii chain isoforms.
Bikoff EK, Kenty G, Van Kaer L. Bikoff EK, et al. J Immunol. 1998 Apr 1;160(7):3101-10. J Immunol. 1998. PMID: 9531264 - Relaxed DM requirements during class II peptide loading and CD4+ T cell maturation in BALB/c mice.
Bikoff EK, Wutz G, Kenty GA, Koonce CH, Robertson EJ. Bikoff EK, et al. J Immunol. 2001 Apr 15;166(8):5087-98. doi: 10.4049/jimmunol.166.8.5087. J Immunol. 2001. PMID: 11290790 - Quality control of MHC class II associated peptides by HLA-DM/H2-M.
Vogt AB, Arndt SO, Hämmerling GJ, Kropshofer H. Vogt AB, et al. Semin Immunol. 1999 Dec;11(6):391-403. doi: 10.1006/smim.1999.0197. Semin Immunol. 1999. PMID: 10625593 Review.
Cited by
- Pathways of antigen processing.
Blum JS, Wearsch PA, Cresswell P. Blum JS, et al. Annu Rev Immunol. 2013;31:443-73. doi: 10.1146/annurev-immunol-032712-095910. Epub 2013 Jan 3. Annu Rev Immunol. 2013. PMID: 23298205 Free PMC article. Review. - A role for HLA-DO as a co-chaperone of HLA-DM in peptide loading of MHC class II molecules.
Kropshofer H, Vogt AB, Thery C, Armandola EA, Li BC, Moldenhauer G, Amigorena S, Hämmerling GJ. Kropshofer H, et al. EMBO J. 1998 Jun 1;17(11):2971-81. doi: 10.1093/emboj/17.11.2971. EMBO J. 1998. PMID: 9606180 Free PMC article. - Positive selection of self-MHC-reactive T cells by individual peptide-MHC class II complexes.
Barton GM, Beers C, deRoos P, Eastman SR, Gomez ME, Forbush KA, Rudensky AY. Barton GM, et al. Proc Natl Acad Sci U S A. 2002 May 14;99(10):6937-42. doi: 10.1073/pnas.102645699. Proc Natl Acad Sci U S A. 2002. PMID: 12011451 Free PMC article. - Invariant chain controls H2-M proteolysis in mouse splenocytes and dendritic cells.
Pierre P, Shachar I, Matza D, Gatti E, Flavell RA, Mellman I. Pierre P, et al. J Exp Med. 2000 Mar 20;191(6):1057-62. doi: 10.1084/jem.191.6.1057. J Exp Med. 2000. PMID: 10727467 Free PMC article. - The nonconventional MHC class II molecule DM governs diabetes susceptibility in NOD mice.
Morgan MA, Muller PS, Mould A, Newland SA, Nichols J, Robertson EJ, Cooke A, Bikoff EK. Morgan MA, et al. PLoS One. 2013;8(2):e56738. doi: 10.1371/journal.pone.0056738. Epub 2013 Feb 13. PLoS One. 2013. PMID: 23418596 Free PMC article.
References
- Harding CV. Intracellular organelles involved in antigen processing and the binding of peptides to class II MHC molecules. Semin Immunol. 1995;7:355–360. - PubMed
- Cresswell P. Invariant chain structure and MHC class II function. Cell. 1996;84:505–507. - PubMed
- Viville S, Neefjes J, Lotteau V, Dierich A, Lemeur M, Ploegh H, Benoist C, Mathis D. Mice lacking the MHC class II–associated invariant chain. Cell. 1993;72:635–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NRSA AI-08903/AI/NIAID NIH HHS/United States
- R37 AI034206/AI/NIAID NIH HHS/United States
- F32 AI008903/AI/NIAID NIH HHS/United States
- AI-34206/AI/NIAID NIH HHS/United States
- R01 AI034206/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous