Bcl-xL is an antiapoptotic regulator for postnatal CNS neurons - PubMed (original) (raw)
Bcl-xL is an antiapoptotic regulator for postnatal CNS neurons
A S Parsadanian et al. J Neurosci. 1998.
Abstract
Bcl-xL is a death-inhibiting member of the Bcl-2/Ced9 family of proteins which either promote or inhibit apoptosis. Gene targeting has revealed that Bcl-xL is required for neuronal survival during brain development; however, Bcl-xL knock-out mice do not survive past embryonic day 13.5, precluding an analysis of Bcl-xL function at later stages of development. Bcl-xL expression is maintained at a high level postnatally in the CNS, suggesting that it may also regulate neuron survival in the postnatal period. To explore functions of Bcl-xL related to neuron survival in postnatal life, we generated transgenic mice overexpressing human Bcl-xL under the control of a pan-neuronal promoter. A line that showed strong overexpression in brainstem and a line that showed overexpression in hippocampus and cortex were chosen for analysis. We asked whether overexpression of Bcl-xL influences neuronal survival in the postnatal period by studying two injury paradigms that result in massive neuronal apoptosis. In the standard neonatal facial axotomy paradigm, Bcl-xL overexpression had substantial effects, with survival of 65% of the motor neurons 7 d after axotomy, as opposed to only 15% in nontransgenic littermates. To investigate whether Bcl-xL regulates survival of CNS neurons in the forebrain, we used a hypoxia-ischemia paradigm in neonatal mice. We show here that hypoxia-ischemia leads to substantial apoptosis in the hippocampus and cortex of wild-type neonatal mice. Furthermore, we show that overexpression of Bcl-xL is neuroprotective in this paradigm. We conclude that levels of Bcl-xL in postnatal neurons may be a critical determinant of their susceptibility to apoptosis.
Figures
Fig. 1.
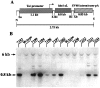
Generation and characterization of_Bcl-x_ L transgenic mice. A, Schematic presentation of the Bcl-x L transgene construct: RI, _Eco_RI; N,_Not_I; S, _Sac_I;Sa, _Sal_I; X,Xho_I; Xb, Xba_I.B, Southern blot analysis of the different transgenic lines. The 6 kb fragment corresponds to the endogenous_Bcl-x L gene. The 0.8 kb fragment corresponds to_Bcl-x L transgene. Lines 7193,7199, and 7194 had the highest copy numbers and were used for further analysis.
Fig. 2.
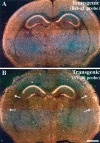
The Bcl-x L transgene is expressed at high levels in the facial motor nucleus of line 7193.A, Section through the brainstem of a transgenic mouse hybridized with a probe for Bcl-x L. This probe detects endogenous and transgenic Bcl-x L mRNA, both of which are expressed throughout the brainstem and facial nucleus. B, Adjacent section from the same animal as in_A_, hybridized with the SV40 probe, which detects only the transgene-derived Bcl-x L mRNA. Note the high levels of expression of SV40 in motor neurons of the facial nucleus (arrows). C, Brainstem section of a wild-type littermate hybridized with the SV40 probe. Note that no signal was detected in the facial nucleus. Scale bar, 0.5 mm.
Fig. 3.
The Bcl-x L transgene is expressed at high levels in the forebrain of line 7194.A, Section through the forebrain of a transgenic mouse hybridized with a probe for Bcl-x L. This probe detects both the endogenous and transgene-derived_Bcl-x_ L. B, Adjacent section from the same brain as in A, hybridized with the SV40 probe, which detects only transgene-derived Bcl-x _L_mRNA. Note the high levels of transgene expression in the cerebral cortex (arrows) and thalamic nuclei. Also note the high expression in CA1, CA2 and CA4 regions of the hippocampus, and the minimal expression in the dentate and CA3 regions. Scale bar, 1 mm.
Fig. 4.
Bcl-xL overexpression protects motor neurons from axotomy-induced cell death. A, Ret-labeled motor neurons in the facial nucleus of a nonaxotomized wild-type mouse. Note the labeling in the medial and lateral (circled) portion of the nucleus. Inset, High-magnification view of Ret labeling in wild-type neurons. B, Ret-labeled motor neurons in the facial nucleus of an axotomized wild-type mouse. Cells in the lateral portion of the nucleus have degenerated (circled), and Ret is no longer detectable except in the medial portion of the nucleus, which is not affected by this lesion paradigm. C, Ret-labeled motor neurons in the facial nucleus of an axotomized transgenic Bcl-x L mouse (line 7193). Many lateral motor neurons survive axotomy (circled), although they are reduced in size.Inset, High-magnification view of Ret labeling in the rescued lateral lateral motor neurons. (Compare the size of motor neurons in the inset in C with the inset_in A.) D, Cresyl violet-stained motor neurons in the facial nucleus of an axotomized transgenic_Bcl-x L mouse. E, High-magnification bright-field view of facial motor neurons 7 d after axotomy in transgenic line 7194. Arrows indicate rescued motor neurons. F, Dark-field view of the same section as in E, labeled with the SV40 probe to detect transgene-derived sequences. Note the high degree of correlation between the rescued motor neurons shown in E and the expression of transgene-derived Bcl-x L shown in_F_ (arrows). Scale bars:A–D, 100 μm; E–F, insets, 25 μm.
Fig. 5.
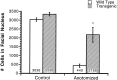
Quantification of facial motor neuron rescue in transgenic line 7193. The bar graph shows numbers of facial motor neurons in control and axotomized wild-type and_Tα1-Bcl-x_ L transgenic mice. Note the large difference in numbers of facial motor neurons that survive axotomy between wild-type and trangenic animals. _Asterisk_indicates statistically significant differences (p < 0.05).
Fig. 6.
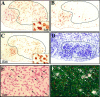
TUNEL labeling after unilateral carotid ligation and exposure to hypoxia. At different time points after hypoxia–ischemia, the cortex ipsilateral to carotid ligation (ischemic cortex) was assessed for the presence of TUNEL-positive cells in P7 mice. A, There were no TUNEL-positive nuclei at 0 hr. B, Occasional TUNEL-positive nuclei began to appear at 6 hr. C, There was an increase in TUNEL labeling at 12 hr, which reached a peak at ∼18 hr (D). Higher-power photomicrographs counterstained with hematoxylin and eosin demonstrate that TUNEL labeling is nuclear. E–F, Arrows point to shrunken TUNEL-positive nuclei (brown) that are also stained with hematoxylin in the cortex 12 hr after hypoxia–ischemia. The arrowheads point to larger, normal-appearing neuronal nuclei adjacent to the smaller, TUNEL-positive nuclei. Scale bars: A–D, 30 μm; E–F, 7.5 μm.
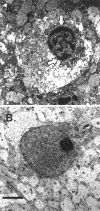
Fig. 7.
Electron microscopy reveals evidence of apoptosis after hypoxic–ischemic injury. A, Example of a cell from the cortex of a P7 mouse brain ipsilateral to carotid ligation, 12 hr after exposure to 8% oxygen. There is condensed chromatin (arrows) within the nucleus. B, There is a normal-appearing neuronal nucleus in the P7 mouse cortex contralateral to carotid ligation 12 hr after exposure to 8% oxygen. Scale bar, 5 μm.
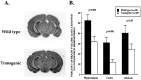
Fig. 8.
Overexpression of Bcl-xL protects the neonatal mouse brain from hypoxic–ischemic insults. A, Coronal sections of P14 mouse brains 1 week after unilateral (left) carotid ligation and exposure to hypoxia for 1 hr at P7. There was significantly less tissue damage in Bcl-x _L_transgenic brains (line 7194) compared with wild-type littermates.B, Quantitative measures of volume loss in transgenic and wild-type animals. The volume of tissue loss in each brain region ipsilateral to carotid ligation (lesioned hemisphere) was compared in each animal with the volume of tissue remaining in the matching brain regions contralateral to carotid ligation (unlesioned hemisphere). The percent volume loss in each structure was determined in each animal, and data are presented as the mean ± SEM.
Similar articles
- cpp32 messenger RNA neosynthesis is induced by fatal axotomy and is not regulated by athanatal Bcl-2 over-expression.
Guarin E, Seuret P, Nef S, de Bilbao F, Nef P, Dubois-Dauphin M. Guarin E, et al. Neuroscience. 1999 May;90(2):653-64. doi: 10.1016/s0306-4522(98)00445-x. Neuroscience. 1999. PMID: 10215167 - Interleukin 3 prevents delayed neuronal death in the hippocampal CA1 field.
Wen TC, Tanaka J, Peng H, Desaki J, Matsuda S, Maeda N, Fujita H, Sato K, Sakanaka M. Wen TC, et al. J Exp Med. 1998 Aug 17;188(4):635-49. doi: 10.1084/jem.188.4.635. J Exp Med. 1998. PMID: 9705946 Free PMC article. - Increased expression of cyclin G1 and p21WAF1/CIP1 in neurons following transient forebrain ischemia: comparison with early DNA damage.
van Lookeren Campagne M, Gill R. van Lookeren Campagne M, et al. J Neurosci Res. 1998 Aug 1;53(3):279-96. doi: 10.1002/(SICI)1097-4547(19980801)53:3<279::AID-JNR2>3.0.CO;2-7. J Neurosci Res. 1998. PMID: 9698156 - Erythropoietin protects neurons against chemical hypoxia and cerebral ischemic injury by up-regulating Bcl-xL expression.
Wen TC, Sadamoto Y, Tanaka J, Zhu PX, Nakata K, Ma YJ, Hata R, Sakanaka M. Wen TC, et al. J Neurosci Res. 2002 Mar 15;67(6):795-803. doi: 10.1002/jnr.10166. J Neurosci Res. 2002. PMID: 11891794 - Unknotting the roles of Bcl-2 and Bcl-xL in cell death.
Kim R. Kim R. Biochem Biophys Res Commun. 2005 Jul 29;333(2):336-43. doi: 10.1016/j.bbrc.2005.04.161. Biochem Biophys Res Commun. 2005. PMID: 15922292 Review.
Cited by
- Delayed cell death signaling in traumatized central nervous system: hypoxia.
Chu D, Qiu J, Grafe M, Fabian R, Kent TA, Rassin D, Nesic O, Werrbach-Perez K, Perez-Polo R. Chu D, et al. Neurochem Res. 2002 Feb;27(1-2):97-106. doi: 10.1023/a:1014858707218. Neurochem Res. 2002. PMID: 11926281 Review. - Expression of cell death-associated proteins in neuronal apoptosis associated with pontosubicular neuron necrosis.
Stadelman C, Mews I, Srinivasan A, Deckwerth TL, Lassmann H, Brück W. Stadelman C, et al. Brain Pathol. 2001 Jul;11(3):273-81. doi: 10.1111/j.1750-3639.2001.tb00398.x. Brain Pathol. 2001. PMID: 11414470 Free PMC article. - Developmental shift of cyclophilin D contribution to hypoxic-ischemic brain injury.
Wang X, Carlsson Y, Basso E, Zhu C, Rousset CI, Rasola A, Johansson BR, Blomgren K, Mallard C, Bernardi P, Forte MA, Hagberg H. Wang X, et al. J Neurosci. 2009 Feb 25;29(8):2588-96. doi: 10.1523/JNEUROSCI.5832-08.2009. J Neurosci. 2009. PMID: 19244535 Free PMC article. - Conditional gene ablation of Stat3 reveals differential signaling requirements for survival of motoneurons during development and after nerve injury in the adult.
Schweizer U, Gunnersen J, Karch C, Wiese S, Holtmann B, Takeda K, Akira S, Sendtner M. Schweizer U, et al. J Cell Biol. 2002 Jan 21;156(2):287-97. doi: 10.1083/jcb.200107009. Epub 2002 Jan 21. J Cell Biol. 2002. PMID: 11807093 Free PMC article. - Activation of nuclear factor kappaB and Bcl-x survival gene expression by nerve growth factor requires tyrosine phosphorylation of IkappaBalpha.
Bui NT, Livolsi A, Peyron JF, Prehn JH. Bui NT, et al. J Cell Biol. 2001 Feb 19;152(4):753-64. doi: 10.1083/jcb.152.4.753. J Cell Biol. 2001. PMID: 11266466 Free PMC article.
References
- Allsopp TE, Wyatt S, Paterson HF, Davies AM. The proto-oncogene bcl-2 can selectively rescue neurotrophic factor-dependent neurons from apoptosis. Cell. 1993;73:295–307. - PubMed
- Bakhshi A, Jensen JP, Goldman P, Wright JJ, McBride OW, Epstein AL, Korsmeyer SJ. Cloning and chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit of 18. Cell. 1985;41:899–906. - PubMed
- Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. bcl-x, a bcl-2 -related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. - PubMed
- Brown AW, Brierley JB. Anoxic-ischemic cell change in rat brain: light microscopic and fine-structural observations. J Neurol Sci. 1972;16:59–84. - PubMed
- Cheng Y, Gidday JM, Yan Q, Shah AR, Holtzman DM. Marked age-dependent neuroprotection by brain-derived neurotrophic factor against neonatal hypoxic-ischemic brain injury. Ann Neurol. 1997;41:521–529. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous