Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries - PubMed (original) (raw)
Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries
A Alvarez-Buylla et al. J Neurosci. 1998.
Abstract
New neurons continue to be born in the ventricular zone (VZ) of the lateral ventricles in the brain of adult birds. On the basis of serial section reconstruction and electron microscopy, we determined that the VZ of the adult canary brain is composed of three main cell types (A, B, and E). Type A cells were never found in contact with the ventricle and had microtubule-rich processes typical of young migrating neurons. Type B cells were organized as a pseudostratified epithelium, all contacted the ventricle, and most had a characteristic single cilium. Type E cells, also in contact with ventricle, were ultrastructurally similar to the mammalian multiciliated ependymal cells. After six injections of [3H]-thymidine (1 every 12 hr), Types A and B cells were found labeled. Type E cells were never [3H]-thymidine labeled. One to two hours after a single injection of [3H]-thymidine, all labeled cells corresponded to Type B cells. At survivals of 5, 24, and 74 hr after [3H]-thymidine injection, the proportion of labeled Type B cells decreased and that of Type A cells increased, indicating that Type B cells were the primary precursors. Most [3H]-labeled nuclei at 1-2 hr after [3H]-thymidine injection were separated from the ventricular cavity, but most of the mitotic cells were adjacent to the ventricle. This observation and measurements of the distance between labeled nuclei and the ventricular surface at 1, 5, 7, and 11 hr after [3H]-thymidine injection indicate that Type B cell nuclei move toward the ventricle to divide. This work reveals the architecture of the VZ in an adult vertebrate brain, identifies the primary precursor of new neurons, and describes nuclear translocation of these precursors during the cell cycle.
Figures
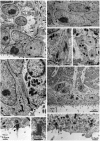
Fig. 1.
A, Hemisection of the adult canary brain at the level of the anterior commissure (AC). The lateral ventricle is indicated by dots, and the approximate locations of regions shown in B–D are indicated by black rectangles. Regions studied in detail are in the dorsal and ventral neurogenic hot spots (Alvarez-Buylla et al., 1990b). H, Hyperstriatum; Hp, hippocampus; N, neostriatum; LPO, lobus parolfactorius; Ot, optic tectum. B, Region of the dorsal neurogenic hot spot with [3H]-thymidine-labeled cells (arrows) in the lateral wall facing hyperstriatum. The ventricle in this region is collapsed and the dorsal wall, facing Hp, has no labeled cells. This animal received six injections of [3H]-thymidine at 12 hr intervals and was killed 1 hr after the last injection. The germinal epithelium is composed of a single layer of flattened cells. C, Region of high proliferation in the ventral hot spot of the same animal as in_B_. The VZ in this region is composed of multiple layers, and many labeled cells (some indicated by arrows) are found in all layers. Few cilia are observed in regions of high proliferation. D, In contrast, regions with many cilia (arrows), such as this one ventral to C, contain fewer labeled cells. E, Electron micrograph of the transition zone between heavily ciliated and relatively unciliated epithelium showing the three major cell types in the VZ of the ventral hotspot: heavily ciliated VZ composed of Type E cells (e) or ependymal cells on the_left_, VZ composed of Type B cells (b) on the right, and two Type A cells (a) deeper in the epithelium. Notice the characteristic ultrastructure of the different cell types (see text for description of corresponding ultrastructures). V, Ventricle lumen. This micrograph is from a region between_C_ and D.
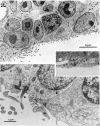
Fig. 2.
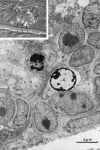
Ultrastructural characteristics of the three main cell types in the adult canary VZ. A, Photomicrograph of Types A and B cells. Type A cells (a) are deeper in the VZ and have very scant cytoplasm. In contrast, Type B cells (b) have a cytoplasm rich in organelles, contact the ventricular lumen (see Results for full description), and frequently show a long basal process (arrows) entering the underlying brain parenchyma. B, Type A (a) cells can be found under Type B (b) or E (not seen here) cells. When cut longitudinally, Type A cells have a spindle shape and tangentially oriented processes (arrows). V, Ventricle. C, A pair of centrioles is observed frequently at the base of Type A (a) cell processes (arrowheads). Some of these centrioles are adjacent to a basal body and an intracellular cilium (large arrow). Processes of Type A cells are linked to neighboring cells by small electron-dense junctional complexes (small arrows). D, Cross section of Type A (a) cell processes (arrows) showing bundles of microtubules. The cell bodies of some of these processes were identified in serial section reconstructions (Table 3, Fig. 3). E, Ultrastructure of apical cytoplasm of Types B (b) and E (e) cells. Both Type B cells contact the ventricle, one of them through a thin process that squeezes between the neighboring B and E cells. Notice the difference in the electron density, number of polyribosomes, and mitochondria between Types B and E cells. Part of the characteristic cilium (arrow) of Type B cells can also be observed in the cell in the middle. V, Ventricle. F, Most apical cytoplasm of Type B cell showing characteristic cilium (arrowhead), basal body (large arrow), and associated centriole (small arrow). In contrast to Type A cells, the centrioles of Type B cells are found close to the ventricular surface; also notice the Golgi apparatus (above the centriole) of Type B cells with flattened sacculae. V, Ventricle. G, Annulate lamellae in the apical cytoplasm of Type B cell. H, Ultrastructure of Type E or ependymal cells (e). Basal processes (asterisks) in Type E cells were electrolucent and rich in intermediate filaments. The luminal surface contains microvilli and clusters of cilia. Three Type A (a) cells are also observed in this micrograph.V, Ventricle. I, Detail of apical cytoplasm in ependymal cells; notice multiple cilia (arrows) and swollen Golgi apparatus sacculae (arrowhead). V, Ventricle.
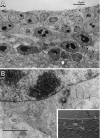
Fig. 3.
Specialized cilium in Type B cells.A, Longitudinal section of one of these cilia (short arrow). In the top left corner is a cross section of a Type E cell cilium (arrow). Notice the centriole at the base of cilium (arrowhead).B, Cross section (arrow) of a Type B cell cilium showing an 8+0 microtubule structure. The_arrowheads_ point to the Golgi apparatus of two Type B cells.
Fig. 4.
Serial section EM analysis of the dorsal (sections_A–J_, region
b
of Fig.1_A_) and ventral (sections K–T, region
c
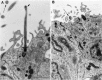
in Fig. 1_A_) part of the lateral wall of the lateral ventricle showing the composition and architecture of the VZ in adult canaries. Each section is separated 2 μm from the next, and the ventricle is to the_left_ in all drawings. In the dorsal VZ the epithelium is thin and flattened. Clusters of Type A cells are next to Type B cells that form a flattened pseudostratified epithelium. Interspersed among Type B cells are small groups of ependymal cells, each with broad exposure to the ventricle. The basal process of some Type E cells can be observed (sections B and F). In the ventral region (K–T) the VZ is thick and rich in Type B cells, with only small islands of ependymal cells. Type B cells are pseudostratified, and a basal process is observed in some of these cells (sections O, R, and_T_). Type A cells in this region are more abundant and are loosely organized as clusters at the interface with the underlying parenchyma. Most of the Type A cells have a thin rim of cytoplasm around them; however, some clusters of Type A cells in this region are larger and have more cytoplasm (e.g., section_M_). These Type A cells were distinguished from Type B cells because they had microtubules, a tangential expansion, or a centriole close to the cell nuclei or in a tangential process.
Fig. 5.
Labeled Type B cell (1) 1 hr after [3H]-thymidine injection.Inset shows labeled cell in Giemsa-stained semithin section. A, Notice how the labeled cell (1) extends a process toward the ventricle and a second process in the opposite direction around an adjacent Type B cell. B, This same cell (1) at higher magnification in a different ultrathin section reaches the ventricular lumen (large arrow), squeezing between processes of other Type B cells. This process contains small junctional complexes with neighboring cells (small arrows) close to its apical end. Notice the characteristic cilium of the neighboring Type B cell (arrowhead).
Fig. 6.
Pair (possibly daughter cells) of [3H]-labeled Type B cells in the dorsal VZ 5 hr after [3H]-thymidine injection.Inset shows the light photomicrograph of a semithin section stained with Giemsa. Notice how cell 2 is exposed to the ventricular lumen (between arrows). The apical borders of this cell are joined by zonulae adherens junctions (arrowheads) to neighboring cells. The dotted line indicates the position of the ventricle. Notice a dark intraventricular cell, possibly a microglia (m).
Fig. 7.
Labeled mitotic cell 5 hr after [3H]-thymidine administration.Inset shows semithin section stained with Giemsa.A, Low-magnification electron micrograph of this cell closely associated with two processes (arrows): one rich in intermediate filaments, probably from an ependymal cell projecting into underlying parenchyma, the other electron-dense, probably from a Type B cell. These processes do not seem to originate in the dividing cell but from closely apposed cells. The exposure of this mitotic cell to the ventricular lumen is indicated by arrowheads.B, At higher magnification, abundant pinocytic vesicles (arrows) and microvilli (arrowheads) but no cilia are seen in the exposed surface of the dividing cell. Notice how this dividing cell establishes junctional complexes (open arrows) with neighboring cells.
Fig. 8.
Two labeled Type A cells (1 and_2_; see autoradiography in Nomarski photomicrograph in the inset) in the ventral hot spot facing a nonciliated section of the ventricular wall 24 hr after [3H]-thymidine administration. A, Low-magnification electron micrograph showing the multilayered VZ. Labeled cells 1 and 2 are among other Type A cells (a) and are separated from the surface of the ventricular lumen by Type B cells (b). Notice the tangential orientation of Type A cells and how the process in cell 2(arrows) seems to reach into the underlying parenchyma.B, High-magnification electron micrograph showing ultrastructural characteristics of labeled Type A cell_1_: a pair of centrioles (arrowheads), abundant polyribosomes, and coated pits (arrows). Notice also the many cross sections of microtubules.
Fig. 9.
Cluster of labeled cells in the ventral hot spot 24 hr after [3H]-thymidine administration. The_inset_ shows the autoradiogram of the semithin section photographed with Nomarski optics. Two labeled cells (_4_and 5) in this group are undergoing apoptosis.
Fig. 10.
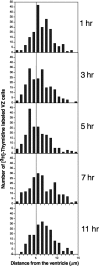
Histograms of distance from the center of [3H]-labeled cells to the surface of the ventricle in the ventral hot spot of adult canaries. Each histogram compounds 200 cells counted in four birds (50 cells/bird). A vertical line at 5 μm distance aids in the comparison of distributions. Three and five hours after [3H]-thymidine injection more cells are <5 μm from the ventricle as compared with after 1, 7, and 11 hr, when most of the cells are to the right of the line.
Fig. 11.
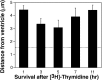
Average distances between the center of the nuclei and the ventricular surface of the 20 [3H]-labeled cells closest to the ventricle in the ventral hot spot of adult canaries. Each bar is the average of four birds. At 3 and 5 hr survivals, a significant (p = 0.02; Mann–Whitney_U_) reduction in this distance was observed compared with 1 or 11 hr survivals. Averages for 1, 7, and 11 hr were not significantly different. One-factor ANOVA also showed significant differences (p < 0.05) between 1 hr and 3 or 5 hr survivals. Likewise, 3 and 5 hr survivals were significantly different from 11 hr survivals. The _dashed line_indicates the average VZ cell radius perpendicular to the ventricle in the ventral hot spot.
Fig. 12.
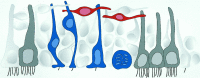
Schematic summary of results. Three main cell types comprise the VZ of adult canaries: Type A (red), Type B (blue), and ependymal cells (gray). Ependymal cells are multiciliated and do not divide. Type B cells are pseudostratified, and all contact the ventricle that has a single short cilium on this lumen. These cells are the primary precursors. Their nuclei migrate from basal to apical VZ to round up and divide adjacent to the ventricular cavity (bottom). Type A cells do not contact the ventricle, are oriented largely parallel to the ventricular surface, and have characteristics of young migrating neurons. Results suggest that Type A cells are derived from Type B cells and may move tangentially to the VZ before radial migration.
Similar articles
- Proliferation "hot spots" in adult avian ventricular zone reveal radial cell division.
Alvarez-Buylla A, Theelen M, Nottebohm F. Alvarez-Buylla A, et al. Neuron. 1990 Jul;5(1):101-9. doi: 10.1016/0896-6273(90)90038-h. Neuron. 1990. PMID: 2369518 - Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain.
Goldman SA, Nottebohm F. Goldman SA, et al. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2390-4. doi: 10.1073/pnas.80.8.2390. Proc Natl Acad Sci U S A. 1983. PMID: 6572982 Free PMC article. - Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment.
Tramontin AD, García-Verdugo JM, Lim DA, Alvarez-Buylla A. Tramontin AD, et al. Cereb Cortex. 2003 Jun;13(6):580-7. doi: 10.1093/cercor/13.6.580. Cereb Cortex. 2003. PMID: 12764031 Review. - The proliferative ventricular zone in adult vertebrates: a comparative study using reptiles, birds, and mammals.
García-Verdugo JM, Ferrón S, Flames N, Collado L, Desfilis E, Font E. García-Verdugo JM, et al. Brain Res Bull. 2002 Apr;57(6):765-75. doi: 10.1016/s0361-9230(01)00769-9. Brain Res Bull. 2002. PMID: 12031273 Review.
Cited by
- Adult Hippocampal Neurogenesis in Different Taxonomic Groups: Possible Functional Similarities and Striking Controversies.
Augusto-Oliveira M, Arrifano GPF, Malva JO, Crespo-Lopez ME. Augusto-Oliveira M, et al. Cells. 2019 Feb 5;8(2):125. doi: 10.3390/cells8020125. Cells. 2019. PMID: 30764477 Free PMC article. Review. - Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions.
Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Shen Q, et al. Cell Stem Cell. 2008 Sep 11;3(3):289-300. doi: 10.1016/j.stem.2008.07.026. Cell Stem Cell. 2008. PMID: 18786416 Free PMC article. - The relationship of neurogenesis and growth of brain regions to song learning.
Kirn JR. Kirn JR. Brain Lang. 2010 Oct;115(1):29-44. doi: 10.1016/j.bandl.2009.09.006. Epub 2009 Oct 23. Brain Lang. 2010. PMID: 19853905 Free PMC article. Review. - Putative adult neurogenesis in two domestic pigeon breeds (Columba livia domestica): racing homer versus utility carneau pigeons.
Mazengenya P, Bhagwandin A, Nkomozepi P, Manger PR, Ihunwo AO. Mazengenya P, et al. Neural Regen Res. 2017 Jul;12(7):1086-1096. doi: 10.4103/1673-5374.211187. Neural Regen Res. 2017. PMID: 28852390 Free PMC article.
References
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–354. - PubMed
- Alvarez-Buylla A, Vicario DS. Simple microcomputer system for mapping tissue sections with the light microscope. J Neurosci Methods. 1988;25:165–173. - PubMed
- Alvarez-Buylla A, Buskirk DR, Nottebohm F. Monoclonal antibody reveals radial glia in adult avian brain. J Comp Neurol. 1987;264:159–170. - PubMed
- Alvarez-Buylla A, Kirn JR, Nottebohm F. Birth of projection neurons in adult avian brain may be related to perceptual or motor learning. Science. 1990a;249:1444–1446. - PubMed
- Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990b;5:101–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical