YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops - PubMed (original) (raw)
YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops
A W Williams et al. J Bacteriol. 1998 Jan.
Abstract
Yersinia pestis produces a set of virulence proteins (Yops and LcrV) that are expressed at high levels and secreted by a type III secretion system (Ysc) upon bacterium-host cell contact, and four of the Yops are vectorially translocated into eukaryotic cells. YopD, YopB, and YopK are required for the translocation process. In vitro, induction and secretion occur at 37 degrees C in the absence of calcium. LcrH (also called SycD), a protein required for the stability and secretion of YopD, had initially been identified as a negative regulator of Yop expression. In this study, we constructed a yopD mutation in both wild-type and secretion-defective (ysc) Y. pestis to determine if the lcrH phenotype could be attributed to the decreased stability of YopD. These mutants were constitutively induced for expression of Yops and LcrV, despite the presence of the secreted negative regulator LcrQ, demonstrating that YopD is involved in negative regulation, regardless of a functioning Ysc system. Normally, secretion of Yops and LcrV is blocked in the presence of calcium. The single yopD mutant was not completely effective in blocking secretion: LcrV was secreted equally well in the presence and absence of calcium, while there was partial secretion of Yops in the presence of calcium. YopD is probably not rate limiting for negative regulation, as increasing levels of YopD did not result in decreased Yop expression. Overexpression of LcrQ in the yopD mutant had no significant effect on Yop expression, whereas increased levels of LcrQ in the parent resulted in decreased levels of Yops. These results indicate that LcrQ requires YopD to function as a negative regulator.
Figures
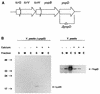
FIG. 1
Construction of a complete Δ_yopD_ mutation in Y. pestis. (A) Schematic representation of the lcrGVHyopBD operon of Y. pestis LCR plasmid pCD1. The positions and directions of the genes of this operon are shown as horizontal arrows. The DNA region deleted to create the yopD mutation is indicated (Δ_yopD_). (B) Immunoblot analysis of YopD and LcrH expressed in Y. pestis KIM8-3002.2 (Δ_yopD_) and Y. pestis KIM8-3002 (parent). Bacteria were grown at 37°C in the absence (−) or presence (+) of 2.5 mM Ca2+. Proteins from equal numbers of cells (0.025 _A_620 unit · ml) were separated by SDS-PAGE in a 15% (wt/vol) polyacrylamide gel. Antibodies were used to detect YopD and LcrH in the soluble (S) (cytoplasmic and periplasmic) fraction, the membrane (M) fraction, and the extracellular (E) fraction. The positions of the molecular mass markers (in kilodaltons) and the LCR-related proteins are indicated.
FIG. 2
Immunoblot analysis of YopM, LcrV, LcrQ, and LcrH expressed in Y. pestis KIM8-3002 (parent) and Y. pestis KIM8-3002.2 (Δ_yopD_). Bacteria were grown at 37°C in the absence (−) or presence (+) of 2.5 mM Ca2+. Proteins from equal numbers of cells (0.025 A_620 unit · ml) were separated by SDS-PAGE in a 12 or 15% (wt/vol) polyacrylamide gel. Antibodies were used to detect YopM, LcrV, LcrQ, and LcrH in both the whole-cell (C) (cytoplasmic, periplasmic, and membrane) fraction and the extracellular (E) fraction. The positions of the LCR-related proteins are indicated. The band corresponding to YopM in the whole-cell fraction of Y. pestis (Δ_yopD) grown in the presence of Ca2+ has a hollow appearance, which occurs when a large quantity of protein is analyzed.
FIG. 3
Immunoblot analysis of YopD, YopM, and LcrQ expressed in the complemented Y. pestis Δ_yopD_ strain (KIM8-3002.2 carrying pAW161) and the parent Y. pestis with overexpression of YopD (KIM8-3002 carrying pAW161). Bacteria were grown at 37°C in both the absence (−) and presence (+) of 2.5 mM Ca2+. Expression of YopD from pAW161 was either uninduced (−) or induced (+) by the addition of arabinose to 0.05% (wt/vol). Proteins from equal numbers of cells (0.025 _A_620 unit · ml) were separated by SDS-PAGE in a 12 (YopD and YopM) or 15% (LcrQ) (wt/vol) polyacrylamide gel. Antibodies were used to detect YopD, YopM, and LcrQ in both the whole-cell (C) (cytoplasmic, periplasmic, and membrane) fraction and the extracellular (E) fraction. The positions of the LCR-related proteins are indicated.
FIG. 4
Immunoblot analysis of YopM, LcrV, and LcrQ expressed in the ysc mutant Y. pestis KIM5-3001.2 (Δ_lcrD_) and the double mutant Y. pestis KIM5-3001.2 (Δ_lcrD ΔyopD_). Bacteria were grown at 37°C in the absence (−) or presence (+) of 2.5 mM Ca2+. Proteins from equal numbers of cells (0.025 _A_620 unit · ml) were separated by SDS-PAGE in a 12 (YopM and LcrV) or 15% (LcrQ) (wt/vol) polyacrylamide gel. Antibodies were used to detect YopM, LcrV, and LcrQ in both the whole-cell (C) (cytoplasmic, periplasmic, and membrane) fraction and the extracellular (E) fraction. The positions of the LCR-related proteins are indicated.
FIG. 5
Immunoblot analysis of LcrQ and YopM expressed in Y. pestis KIM8-3002 (parent) and Y. pestis KIM8-3002.2 (Δ_yopD_) with overexpression of LcrQ from plasmid pAW162 in both strains. Bacteria were grown at 37°C in the absence (−) or presence (+) of 2.5 mM Ca2+. Expression of LcrQ from pAW161 was either uninduced (−) or induced (+) by the addition of arabinose to 0.2% (wt/vol). Proteins from equal numbers of cells (0.025 _A_620 unit · ml) were separated by SDS-PAGE in a 12 (YopM) or 15% (LcrQ) (wt/vol) polyacrylamide gel. Antibodies were used to detect LcrQ and YopM in both the whole-cell (C) (cytoplasmic, periplasmic, and membrane) fraction and the extracellular (E) fraction. The positions of the LCR-related proteins are indicated.
FIG. 6
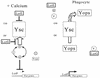
Model for regulation of the LCR in Y. pestis. In the presence of Ca2+ (+ Calcium), LcrE and LcrG block the Yop secretion system (Ysc) at the cell surface and in the cytoplasm, respectively. The secretion block prevents the secretion of LcrQ (Q) and YopD (D). These two proteins work in concert to cause a down-regulation of Yop expression. There may be other components (?) required that have not yet been identified. In the absence of Ca2+ and in vivo when the bacteria contact a eukaryotic cell, LcrE is released from the cell surface. This relieves the secretion block and allows secretion of both LcrQ and YopD, which results in induction of the yop genes, including lcrV. The increase in LcrV (V) levels titrates LcrG away from the Ysc, resulting in full LCR induction and secretion. YopD and YopB (B) are then involved in translocating the Yops into the eukaryotic cell. OM, outer membrane; IM, inner membrane.
Similar articles
- The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG.
Nilles ML, Fields KA, Straley SC. Nilles ML, et al. J Bacteriol. 1998 Jul;180(13):3410-20. doi: 10.1128/JB.180.13.3410-3420.1998. J Bacteriol. 1998. PMID: 9642196 Free PMC article. - LcrQ and SycH function together at the Ysc type III secretion system in Yersinia pestis to impose a hierarchy of secretion.
Wulff-Strobel CR, Williams AW, Straley SC. Wulff-Strobel CR, et al. Mol Microbiol. 2002 Jan;43(2):411-23. doi: 10.1046/j.1365-2958.2002.02752.x. Mol Microbiol. 2002. PMID: 11985718 - The type III secretion chaperone LcrH co-operates with YopD to establish a negative, regulatory loop for control of Yop synthesis in Yersinia pseudotuberculosis.
Francis MS, Lloyd SA, Wolf-Watz H. Francis MS, et al. Mol Microbiol. 2001 Nov;42(4):1075-93. doi: 10.1046/j.1365-2958.2001.02702.x. Mol Microbiol. 2001. PMID: 11737648 - The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells.
Cornelis GR, Wolf-Watz H. Cornelis GR, et al. Mol Microbiol. 1997 Mar;23(5):861-7. doi: 10.1046/j.1365-2958.1997.2731623.x. Mol Microbiol. 1997. PMID: 9076724 Review. - The Yersinia Ysc-Yop virulence apparatus.
Cornelis GR. Cornelis GR. Int J Med Microbiol. 2002 Feb;291(6-7):455-62. doi: 10.1078/1438-4221-00153. Int J Med Microbiol. 2002. PMID: 11890544 Review.
Cited by
- Calcium and iron regulate swarming and type III secretion in Vibrio parahaemolyticus.
Gode-Potratz CJ, Chodur DM, McCarter LL. Gode-Potratz CJ, et al. J Bacteriol. 2010 Nov;192(22):6025-38. doi: 10.1128/JB.00654-10. Epub 2010 Sep 17. J Bacteriol. 2010. PMID: 20851895 Free PMC article. - Developing Cyclic Peptomers as Broad-Spectrum Type III Secretion System Inhibitors in Gram-Negative Bacteria.
Lam HN, Lau T, Lentz A, Sherry J, Cabrera-Cortez A, Hug K, Lalljie A, Engel J, Lokey RS, Auerbuch V. Lam HN, et al. Antimicrob Agents Chemother. 2021 Jun 17;65(7):e0169020. doi: 10.1128/AAC.01690-20. Epub 2021 Jun 17. Antimicrob Agents Chemother. 2021. PMID: 33875435 Free PMC article. - Inflammasome activation in response to the Yersinia type III secretion system requires hyperinjection of translocon proteins YopB and YopD.
Zwack EE, Snyder AG, Wynosky-Dolfi MA, Ruthel G, Philip NH, Marketon MM, Francis MS, Bliska JB, Brodsky IE. Zwack EE, et al. mBio. 2015 Feb 17;6(1):e02095-14. doi: 10.1128/mBio.02095-14. mBio. 2015. PMID: 25691590 Free PMC article. - LcrV of Yersinia pestis enters infected eukaryotic cells by a virulence plasmid-independent mechanism.
Fields KA, Straley SC. Fields KA, et al. Infect Immun. 1999 Sep;67(9):4801-13. doi: 10.1128/IAI.67.9.4801-4813.1999. Infect Immun. 1999. PMID: 10456934 Free PMC article.
References
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1989. Analysis of proteins; p. 10.2.30.
- Beiting, M., G. V. Plano, and S. C. Straley. Unpublished data.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources