Regulation of the human proliferating cell nuclear antigen promoter by the adenovirus E1A-associated protein p107 - PubMed (original) (raw)
Regulation of the human proliferating cell nuclear antigen promoter by the adenovirus E1A-associated protein p107
B H Lee et al. J Virol. 1998 Feb.
Abstract
The adenovirus E1A 243R oncoprotein is capable of transactivating the expression of the human proliferating cell nuclear antigen (PCNA) promoter. Mutational analysis of the E1A 243R protein suggested that both its p300/CBP- and p107-binding regions are required for optimal induction of the PCNA promoter (C. Kannabiran, G. F. Morris, C. Labrie, and M. B. Mathews, J. Virol. 67:425-437, 1993). We show that overexpression of p107 antagonizes the induction of PCNA by E1A 243R in transient expression assays. This inhibition is largely independent of p107's ability to interact with E1A 243R, because p107 mutants unable to bind to E1A 243R retain the ability to repress the E1A-activated PCNA promoter. Electrophoretic mobility shift assays with the PCNA promoter detected the presence of p107 in one of the major DNA-protein complexes, EH1, formed with HeLa cell nuclear extracts. Promoter mutations that disrupt the formation of complex EH1 abrogated p107's ability to reverse E1A 243R-induced PCNA expression. The same mutations characterize a sequence important for the binding of transcription factor RFX1 (C. Labrie, G. F. Morris, and M. B. Mathews, Nucleic Acids Res. 23:3732-3741, 1995), implying that p107 antagonizes E1A 243R-induced PCNA expression through this RFX1-binding site. Our data are suggestive of a novel cooperative mechanism for transactivation of PCNA expression, in which E1A 243R relieves transcriptional repression exerted by p107 on the promoter.
Figures
FIG. 1
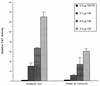
p107 inhibits E1A 243R-induced PCNA-CAT activity. PCNA−87 CAT (10 μg) reporter was transfected into HeLa cells with either pCMV12S.FS (0.5 μg) or increasing amounts of pCMV12S (E1A 243R) plasmid (0.1, 0.5, or 2.5 μg) and a constant amount (2 μg) of wild-type pCMV107 expression plasmid. The results are the average of two independent transfections performed in duplicate with standard deviations indicated.
FIG. 2
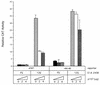
p107 antagonizes E1A 243R-induced PCNA expression independent of its E1A-binding ability. Wild-type (WT) PCNA−87 CAT plasmid reporter (10 μg) was cotransfected into HeLa cells with either pCMV12S.FS as a control or pCMV12S (E1A 243R) and increasing amounts (0, 2, 4, or 6 μg) of either wild-type pCMV107 or mutant pCMV107DE expression plasmids. CAT activity was corrected for β-galactosidase activity generated from a cotransfected reporter plasmid and expressed as the means ± the standard deviation relative to the level of CAT activity obtained by cotransfection of wild-type PCNA−87 CAT with pCMV12S.FS. These data represent the means of three independent transfections performed in duplicate.
FIG. 3
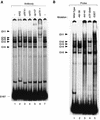
EMSA complex EH1 contains both p107 and RFX1. (A) An end-labeled PCNA promoter fragment from −87 to +62 relative to the transcription initiation site (EH87) was incubated with 5 μg of HeLa cell nuclear extract, and the protein-DNA complexes were resolved on a 4.5% native polyacrylamide gel. Extracts were incubated in the absence (lanes 1 and 7) or the presence of antibodies (α) against either RFX1 (lane 3), p53 (lane 4), p107 (lane 5), or ATF-1 (lane 6). As a control, nuclear extract was incubated with normal rabbit serum (NRS) (lane 2). Complexes EH1 to EH5 are denoted in order of increasing mobility. (B) Formation of complex EH1 depends on an intact RFX1 site. Probes derived from the wild-type PCNA−87 CAT construct (EH87) or from promoter constructs harboring mutations from −50 to −47 (EH ATF-BAM), −46 to −39 (EH −46/−39), −44 to −44 (EH −44/−40), or a G-to-T point mutation at position −53 (EH −53GT) were incubated with HeLa nuclear extract (5 μg), and the protein-DNA complexes were resolved in a native gel.
FIG. 4
p107 inhibition of E1A 243R-induced PCNA expression is dependent on the RFX1-binding site. HeLa cells were transfected with either wild-type PCNA−87 CAT or −44/−40 CAT reporter plasmid (10 μg), pCMV12S.FS or pCMV12S, and increasing amounts of pCMV107 (0, 2, or 4 μg) or pCMV107DE (0, 2, 4, or 6 μg) expression plasmid. CAT activity was calculated as described in the legend to Fig. 1 and represents the average of three independent experiments performed in duplicate with standard deviations indicated.
FIG. 5
Inhibition of E1A-induced PCNA activity by p107DE is also dependent upon RFX1-binding site sequences. HeLa cells were transfected with either wild-type (WT) PCNA−87 CAT or −44/−40 CAT reporter plasmid (10 μg), pCMV12S.FS or pCMV12S, and increasing amounts of pCMV107DE (0, 2, 4, or 6 μg) expression plasmid. CAT activity was calculated as described in the legend to Fig. 1 and represents the average of three independent experiments performed in duplicate with standard deviations indicated.
FIG. 6
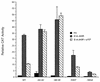
Mutations that specifically affect the RFX1-binding site abrogate reversal of E1A 243R-induced PCNA expression by p107. Wild-type (WT) and mutant (−44/−40, −46/−39, −53GT, −56GA CAT) PCNA-CAT reporter constructs (10 μg) were cotransfected with either 0.5 μg of pCMV12S.FS (control) or pCMV12S (E1A 243R) and with or without pCMV107 expression plasmid (2 μg). The fold increase ± standard deviation in CAT expression was normalized for β-galactosidase activity and represents the average of three independent experiments done in duplicate.
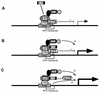
FIG. 7
Model for activation of the human PCNA promoter by E1A 243R. Schematic diagram of the minimal PCNA-E1A-responsive promoter is illustrated with the _cis_-acting PERE boxed in gray, the RFX1-binding site, and the cellular factors indicated. A possible mechanism by which E1A 243R might transactivate the PCNA promoter is depicted sequentially. (A) E1A 243R first targets the PCNA promoter at the PERE site via a CBP-CREB-PERE pathway (37). (B) The binding between E1A and CBP activates the promoter by itself through an interaction between CBP and components of the general transcription machinery (general transcription factors [GTFs]). (C) E1A 243R increases PCNA promoter expression by mediating the relief of a transcriptional repression exerted on the promoter by p107. Note that the events depicted in panels B and C might take place simultaneously.
Similar articles
- Involvement of RFX1 protein in the regulation of the human proliferating cell nuclear antigen promoter.
Liu M, Lee BH, Mathews MB. Liu M, et al. J Biol Chem. 1999 May 28;274(22):15433-9. doi: 10.1074/jbc.274.22.15433. J Biol Chem. 1999. PMID: 10336433 - Transcription factors RFX1/EF-C and ATF-1 associate with the adenovirus E1A-responsive element of the human proliferating cell nuclear antigen promoter.
Labrie C, Lee BH, Mathews MB. Labrie C, et al. Nucleic Acids Res. 1995 Sep 25;23(18):3732-41. doi: 10.1093/nar/23.18.3732. Nucleic Acids Res. 1995. PMID: 7479004 Free PMC article. - A complex promoter element mediates transactivation of the human proliferating cell nuclear antigen promoter by the 243-residue adenovirus E1A oncoprotein.
Labrie C, Morris GF, Mathews MB. Labrie C, et al. Mol Cell Biol. 1993 Mar;13(3):1697-707. doi: 10.1128/mcb.13.3.1697-1707.1993. Mol Cell Biol. 1993. PMID: 8095093 Free PMC article. - Growth regulation of the PCNA gene.
Baserga R. Baserga R. J Cell Sci. 1991 Apr;98 ( Pt 4):433-6. doi: 10.1242/jcs.98.4.433. J Cell Sci. 1991. PMID: 1677646 Review. No abstract available.
Cited by
- Autorepression of rfx1 gene expression: functional conservation from yeast to humans in response to DNA replication arrest.
Lubelsky Y, Reuven N, Shaul Y. Lubelsky Y, et al. Mol Cell Biol. 2005 Dec;25(23):10665-73. doi: 10.1128/MCB.25.23.10665-10673.2005. Mol Cell Biol. 2005. PMID: 16287876 Free PMC article. - RFX1: a promising therapeutic arsenal against cancer.
Issac J, Raveendran PS, Das AV. Issac J, et al. Cancer Cell Int. 2021 May 8;21(1):253. doi: 10.1186/s12935-021-01952-6. Cancer Cell Int. 2021. PMID: 33964962 Free PMC article. Review. - Characterization of dRFX2, a novel RFX family protein in Drosophila.
Otsuki K, Hayashi Y, Kato M, Yoshida H, Yamaguchi M. Otsuki K, et al. Nucleic Acids Res. 2004 Oct 19;32(18):5636-48. doi: 10.1093/nar/gkh895. Print 2004. Nucleic Acids Res. 2004. PMID: 15494451 Free PMC article. - Genetic alterations of the retinoblastoma-related gene RB2/p130 identify different pathogenetic mechanisms in and among Burkitt's lymphoma subtypes.
Cinti C, Leoncini L, Nyongo A, Ferrari F, Lazzi S, Bellan C, Vatti R, Zamparelli A, Cevenini G, Tosi GM, Claudio PP, Maraldi NM, Tosi P, Giordano A. Cinti C, et al. Am J Pathol. 2000 Mar;156(3):751-60. doi: 10.1016/s0002-9440(10)64941-3. Am J Pathol. 2000. PMID: 10702389 Free PMC article. - Downmodulation of E1A protein expression as a novel strategy to design cancer-selective adenoviruses.
Jiang H, Alemany R, Gomez-Manzano C, Medrano DR, Lemoine MG, Olson MV, Alonso MM, Lee OH, Conrad CC, Yung WK, Fueyo J. Jiang H, et al. Neoplasia. 2005 Aug;7(8):723-9. doi: 10.1593/neo.04793. Neoplasia. 2005. PMID: 16207474 Free PMC article.
References
- Arany Z, Sellers W R, Livingston D M, Eckner R. E1A-associated p300 and CREB-associated CBP belong to a conserved family of coactivators. Cell. 1994;77:799–800. - PubMed
- Arany Z, Newsome D, Oldread E, Livingston D M, Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature. 1995;374:81–84. - PubMed
- Bayley S T, Mymryk J S. Adenovirus E1A proteins and transformation. Int J Oncol. 1994;5:425–444. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous