Regulation of interferon-induced protein kinase PKR: modulation of P58IPK inhibitory function by a novel protein, P52rIPK - PubMed (original) (raw)
Regulation of interferon-induced protein kinase PKR: modulation of P58IPK inhibitory function by a novel protein, P52rIPK
M Gale Jr et al. Mol Cell Biol. 1998 Feb.
Abstract
The cellular response to environmental signals is largely dependent upon the induction of responsive protein kinase signaling pathways. Within these pathways, distinct protein-protein interactions play a role in determining the specificity of the response through regulation of kinase function. The interferon-induced serine/threonine protein kinase, PKR, is activated in response to various environmental stimuli. Like many protein kinases, PKR is regulated through direct interactions with activator and inhibitory molecules, including P58IPK, a cellular PKR inhibitor. P58IPK functions to represses PKR-mediated phosphorylation of the eukaryotic initiation factor 2alpha subunit (eIF-2alpha) through a direct interaction, thereby relieving the PKR-imposed block on mRNA translation and cell growth. To further define the molecular mechanism underlying regulation of PKR, we have utilized an interaction cloning strategy to identify a novel cDNA encoding a P58IPK-interacting protein. This protein, designated P52rIPK, possesses limited homology to the charged domain of Hsp90 and is expressed in a wide range of cell lines. P52rIPK and P58IPK interacted in a yeast two-hybrid assay and were recovered as a complex from mammalian cell extracts. When coexpressed with PKR in yeast, P58IPK repressed PKR-mediated eIF-2alpha phosphorylation, inhibiting the normally toxic and growth-suppressive effects associated with PKR function. Conversely, introduction of P52rIPK into these strains resulted in restoration of both PKR activity and eIF-2alpha phosphorylation, concomitant with growth suppression due to inhibition of P58IPK function. Furthermore, P52rIPK inhibited P58IPK function in a reconstituted in vitro PKR-regulatory assay. Our results demonstrate that P58IPK is inhibited through a direct interaction with P52rIPK which, in turn, results in upregulation of PKR activity. Taken together, our data describe a novel protein kinase-regulatory system which encompasses an intersection of interferon-, stress-, and growth-regulatory pathways.
Figures
FIG. 1
P52rIPK possesses homology to the charged domain of Hsp90. (A) Nucleotide and deduced amino acid sequences of the 5′ UTR and open reading frame in the P52rIPK cDNA. Nucleotide and amino acid positions are indicated at the left. Position 1 denotes the initiator methionine codon (boxed); the asterisk denotes the site of translation termination. (B) Structural representation of P52rIPK and comparison with Hsp90. The Hsp90 homology domain of P52rIPK (aa 86 to 200) and the homologous charged domain of human Hsp90 (aa 170 to 300 [37]) are indicated in black (top) and are shown in an amino acid sequence alignment (bottom). Identical residues are indicated by a vertical line; double and single dots indicate conservative and less conservative amino acid replacements, respectively. Homology scores show 24% amino acid identity with 48% amino acid similarity over the region shown. (C) Hydropathy profile of the P52rIPK amino acid sequence. Positive (hydrophobic) amino acid sequences are represented by peaks extending above the neutral plane (dashed line). The bar indicates the Hsp90 homology domain.
FIG. 2
Expression of P52rIPK in mammalian cells. (A) Northern blot analysis of P52rIPK mRNA expression in HeLa cells cultured in the presence (lane 1) or absence (lane 2) of IFN. The 4.2-kb P52rIPK mRNA is indicated by the arrow at the right. Each lane contains 5 μg of poly(A)+ RNA. Gel loading was confirmed by sequentially hybridizing the same blot to probes specific for actin and glyceraldehyde dehydrogenase (gapdh) mRNAs. To confirm the induction of gene expression due to IFN treatment, the same blot was probed with 32P-labeled PKR cDNA. Positions of RNA size standards are indicated in kilobases. (B) Immunoblot analysis. Fifty micrograms of HeLa cell extract was separated by SDS-PAGE, blotted to membranes, and incubated with preimmune (Pre) rabbit serum (lane 1) or α-P52rIPK immune serum (lane 2). The arrow indicates the position of P52rIPK. The faint lower band in lane 2 corresponds to a background band recognized by rabbit serum. Positions of protein standards are shown. (C) Immunoprecipitation (IP) analysis. Reticulocyte lysate (retic) in vitro translation reaction mixtures containing [35S]methionine-labeled P52rIPK (lanes 1 and 2) or extracts from 106 HeLa cells metabolically labeled with [35S]-methionine (lanes 3 and 4) were immunoprecipitated with preimmune rabbit serum (P) (lanes 2 and 4) or α-P52rIPK immune serum (I) (lanes 1 and 3). Shown is an autoradiogram of immunoprecipitates separated by SDS-PAGE. The arrow indicates the position of P52rIPK.
FIG. 3
P52rIPK interacts with P58IPK in vivo. (A) Histidine reporter assay of yeast two-hybrid strains. Hf7c yeast strains harboring GAL4 AD and DNA BD plasmids expressing AD-PKR and BD-P58IPK (controls; position 1), AD-vector (AD-V) (control) and BD-P58IPK (position 2), AD-P52rIPK and BD-P58IPK (position 3), AD-P52rIPK and BD-vector (control; position 4), AD-GAL4 wild type (control) and BD-vector (position 5), and AD-vector and BD-vector (control; position 6) were replica plated in the presence (+ His) or absence (−His) of histidine, incubated for 5 days, and scored for growth. Growth on medium lacking histidine is indicative of a two-hybrid protein interaction. (B) Analysis of β-galactosidase activity. Strain Hf7c was cotransformed with expression plasmids encoding the indicated combination of AD and BD fusion proteins and patch-plated onto medium containing histidine in the presence of β-galactosidase substrate. After 3 days of growth, patches were scored for color development. Induction of the LacZ reporter (dark patch) is indicative of β-galactosidase activity and a two-hybrid protein interaction. The top row shows strains expressing AD-P52rIPK and (from left to right) BD-vector, BD-SV40 T antigen (BD-T ag); BD-lamin, and BD-P58IPK. The bottom row, left, shows strains expressing BD-P58IPK with AD-vector or AD-SV40 T antigen. The bottom row, right, shows strain Hf7c expressing the wild-type (wt) GAL4 protein. In this analysis and that shown in panel A, AD and BD fusion protein expression was confirmed by immunoblot analysis (data not shown).
FIG. 4
P52rIPK inhibits P58IPK in vitro. Purified PKR (2 pmol) was mixed with buffer alone (lane 1) or buffer containing 2 pmol of GST-P58IPK either alone (lane 2) or preincubated with increasing amounts of GST (0.1, 2.0, or 5.0 pmol [lanes 3 to 5, respectively]) or GST-P52rIPK (0.1, 2.0, or 5.0 pmol [lanes 6 to 8, respectively]). To assess PKR activity, protein kinase assays were carried out in the presence of a histone HIIA substrate and [γ-32P]ATP. PKR-phosphorylated histones (arrow) were separated by SDS-PAGE and visualized by autoradiography. The level of PKR activity (histone phosphorylation) was quantitated by scanning laser densitometry and is presented below each lane as percent activity relative to that of the input control (lane 1).
FIG. 5
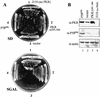
P58IPK represses PKR function in vivo. (A) Yeast growth analysis. Yeast strain RY1-1 was transformed with the 2μm expression plasmid pEMBLYex4 (vector; position 3), pYex-PKR Δ295–300 (PKR Δ295–300; position 2), or pYex-P58IPK (P58IPK; position 4) and streaked onto uracil-deficient medium containing 2% dextrose (SD) or 10% galactose (SGAL). To control for the specificity of growth effects due to PKR or P58IPK expression, the _gcn2_Δ isogenic control strain, J110, harboring vector alone (position 1) was included in all analyses. (B) Protein expression. Lanes 1 to 3 contain extracts from the RY1-1 strains shown in panel A harboring pYex-P58IPK (lane 1), pEMBLYex4 alone (lane 2), or pYex-PKR Δ295–300 (lane 3). An extract from the J110 parental control strain harboring pEMBLYex4 is shown in lane 4. Strains were grown for 5 h in galactose-containing liquid medium, and extracts were prepared as described in Materials and Methods. Proteins (25 μg) were separated by SDS-PAGE and subjected to immunoblot analysis. Panels show the same blot probed sequentially with antibodies specific to PKR, P58IPK, or actin.
FIG. 6
P52rIPK inhibits P58IPK to restore PKR function in vivo. (A) Yeast growth analysis. RY1-1 harboring the indicated Ura and Trp 2μm expression plasmids was plated onto uracil- and tryptophan-deficient dextrose medium (SD) or inducing galactose medium (SGAL) and incubated at 30°C for 7 days. Plasmid combinations for each strain: pYex-P58IPK–pYX233 (P58IPK/v; position 1), pYex-pYX233 (v/v; position 2), pYex–pYX-P52 (v/P52rIPK; position 3), and pYex-P58IPK–pYX-P52 (P58IPK/P52rIPK; position 4). (B) Protein expression. Extracts were prepared from the yeast strains shown in panel A, which were grown for 7 h in galactose-containing liquid medium. Twenty-five micrograms of protein was separated by SDS-PAGE and subjected to immunoblot analysis. Shown is a single blot probed with antibodies specific to PKR, P58IPK, P52rIPK, or actin. Lanes represent extracts from RY1-1 harboring the plasmid combinations pYex-P58IPK–pYX233 (P58IPK/v; lane 1), pYex-pYX233 (v/v; lane 2), pYex-P58IPK–pYX-P52 (P58IPK/P52rIPK; lane 3), and pEMBLYex4–pYX-P52 (v/P52rIPK; lane 4). The arrow indicates the position of P52rIPK, which migrates above a background band found in all lanes.
FIG. 7
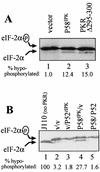
P52rIPK and P58IPK regulate PKR-mediated eIF-2α phosphorylation. Protein extracts (16 μg) from yeast strains cultured in galactose-containing selective liquid medium for 9 h (A) or 5 h (B), were subjected to vertical gel isoelectric focusing and anti-eIF-2α immunoblot analysis. For each panel, the lower arrow indicates the position of hypophosphorylated eIF-2α. The upper arrow denotes the position of hyperphosphorylated eIF-2α, which is phosphorylated on serine 51 by PKR (22). The percentage of hypophosphorylated eIF-2α is presented beneath each lane. (A) Analysis of RY1-1 strains shown in Fig. 5, harboring pEMBLYex4 (vector; lane 1), pYex-P58IPK (lane 2), or pYex-PKR Δ295–300 (lane 3). (B) Analysis of the isogenic control strain J110 harboring pEMBLYex4 (lane 1) and the RY1-1 strains shown in Fig. 6, which harbor the plasmid combinations of pEMBLYex4-pYX233 (v/v; lane 2), pEMBLYex4–pYX-P52 (v/P52rIPK; lane 3), pYex-P58IPK–pYX233 (P58IPK/v; lane 4), and pYex-P58IPK–pYX-P52 (P58/P52; lane 5).
FIG. 8
P58IPK and P52rIPK form a complex in mammalian cell extracts. P58IPK and P52rIPK were translated in vitro in the presence (hot) or absence (cold) of [35S]methionine by using a rabbit reticulocyte lysate translation system. Hot translation reactions were immunoprecipitated from reticulocyte extracts individually (lanes 1 to 4, 6, and 7) or as a mixture with the reciprocal cold translation extract (lanes 5 and 8), boiled in reducing sample buffer, and separated by SDS-PAGE. By this method, coimmunoprecipitating proteins will migrate through the gel independently based upon protein mass. The relative positions of labeled proteins after electrophoresis were determined by autoradiography of the dried gel. Lanes 1 and 2, preimmune serum (Pre) immunoprecipitations (IP) of hot P52rIPK and P58IPK, respectively; lanes 3 to 5, α-P52rIPK immunoprecipitations of hot P52rIPK (lane 3) and of hot P58IPK alone or in the presence of cold P52rIPK (lanes 4 and 5, respectively); lanes 6 to 8, α-P58IPK immunoprecipitations of hot P58IPK (lane 7) and of hot P52rIPK alone or mixed with lysates containing cold P58IPK (lanes 6 and 8, respectively). Arrows indicate the positions of P58IPK and P52rIPK.
FIG. 9
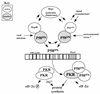
Regulation of PKR. Two distinct cellular pathways of PKR regulation are defined by P52rIPK and Hsp40, both of which converge upon P58IPK to modulate PKR function. P58IPK is regulated through the formation of independent inhibitory complexes with P52rIPK and Hsp40 (58). Cellular stress, including virus infection (55, 56), may induce dissociation of the P58IPK-Hsp40 inhibitory complex. Possibly representing a protein signaling motif, the Hsp90 homology domain of P52rIPK may participate in signal transduction processes induced by exposure to environmental stress or mediated through specific signaling cascades which result in dissociation of the P58IPK-P52rIPK complex. Once released from its inhibitor, P58IPK forms a dimer or higher-ordered homotypic complex (31) competent to bind and disrupt active PKR dimers (lower) (85). Inhibition of PKR results in a block in PKR-mediated eIF-2α phosphorylation, stimulation of protein synthesis, and concomitant repression of PKR tumor suppressor function (lower middle and lower right) (1, 74, 86). P58IPK-mediated inhibition of PKR may also confer alterations in PKR-mediated signal transduction processes (50, 51, 89). Finally, P52rIPK and Hsp40 may interact with P58IPK as regulatory components of PKR-independent processes which lead to alterations in Hsp40 and/or P52rIPK function (upper). Other stress response proteins which regulate Hsp40, including heat shock proteins and molecular chaperones (19), could participate in both PKR-dependent and -independent regulatory pathways.
Similar articles
- Interaction of the interferon-induced PKR protein kinase with inhibitory proteins P58IPK and vaccinia virus K3L is mediated by unique domains: implications for kinase regulation.
Gale M Jr, Tan SL, Wambach M, Katze MG. Gale M Jr, et al. Mol Cell Biol. 1996 Aug;16(8):4172-81. doi: 10.1128/MCB.16.8.4172. Mol Cell Biol. 1996. PMID: 8754816 Free PMC article. - P52rIPK regulates the molecular cochaperone P58IPK to mediate control of the RNA-dependent protein kinase in response to cytoplasmic stress.
Gale M Jr, Blakely CM, Darveau A, Romano PR, Korth MJ, Katze MG. Gale M Jr, et al. Biochemistry. 2002 Oct 1;41(39):11878-87. doi: 10.1021/bi020397e. Biochemistry. 2002. PMID: 12269832 - The molecular chaperone hsp40 regulates the activity of P58IPK, the cellular inhibitor of PKR.
Melville MW, Hansen WJ, Freeman BC, Welch WJ, Katze MG. Melville MW, et al. Proc Natl Acad Sci U S A. 1997 Jan 7;94(1):97-102. doi: 10.1073/pnas.94.1.97. Proc Natl Acad Sci U S A. 1997. PMID: 8990167 Free PMC article. - P58IPK, a novel cochaperone containing tetratricopeptide repeats and a J-domain with oncogenic potential.
Melville MW, Katze MG, Tan SL. Melville MW, et al. Cell Mol Life Sci. 2000 Feb;57(2):311-22. doi: 10.1007/PL00000692. Cell Mol Life Sci. 2000. PMID: 10766025 Free PMC article. Review. - The eIF-2alpha kinases and the control of protein synthesis.
de Haro C, Méndez R, Santoyo J. de Haro C, et al. FASEB J. 1996 Oct;10(12):1378-87. doi: 10.1096/fasebj.10.12.8903508. FASEB J. 1996. PMID: 8903508 Review.
Cited by
- Missing and overexpressing proteins in domestic cat oocytes following vitrification and in vitro maturation as revealed by proteomic analysis.
Turathum B, Roytrakul S, Changsangfa C, Sroyraya M, Tanasawet S, Kitiyanant Y, Saikhun K. Turathum B, et al. Biol Res. 2018 Aug 20;51(1):27. doi: 10.1186/s40659-018-0176-5. Biol Res. 2018. PMID: 30124164 Free PMC article. - Protein folding and quality control in the ER.
Araki K, Nagata K. Araki K, et al. Cold Spring Harb Perspect Biol. 2011 Nov 1;3(11):a007526. doi: 10.1101/cshperspect.a007526. Cold Spring Harb Perspect Biol. 2011. PMID: 21875985 Free PMC article. Review. - Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation.
Gale M Jr, Blakely CM, Kwieciszewski B, Tan SL, Dossett M, Tang NM, Korth MJ, Polyak SJ, Gretch DR, Katze MG. Gale M Jr, et al. Mol Cell Biol. 1998 Sep;18(9):5208-18. doi: 10.1128/MCB.18.9.5208. Mol Cell Biol. 1998. PMID: 9710605 Free PMC article. - Proteome-wide analysis reveals an age-associated cellular phenotype of in situ aged human fibroblasts.
Waldera-Lupa DM, Kalfalah F, Florea AM, Sass S, Kruse F, Rieder V, Tigges J, Fritsche E, Krutmann J, Busch H, Boerries M, Meyer HE, Boege F, Theis F, Reifenberger G, Stühler K. Waldera-Lupa DM, et al. Aging (Albany NY). 2014 Oct;6(10):856-78. doi: 10.18632/aging.100698. Aging (Albany NY). 2014. PMID: 25411231 Free PMC article. - Disruption of the ZFP574-THAP12 complex suppresses B cell malignancies in mice.
Zhong X, Moresco JJ, SoRelle JA, Song R, Jiang Y, Nguyen MT, Wang J, Bu CH, Moresco EMY, Beutler B, Choi JH. Zhong X, et al. Proc Natl Acad Sci U S A. 2024 Jul 30;121(31):e2409232121. doi: 10.1073/pnas.2409232121. Epub 2024 Jul 24. Proc Natl Acad Sci U S A. 2024. PMID: 39047044 Free PMC article.
References
- Bartel P L, Fields S. Analyzing protein-protein interactions using the two-hybrid system. Methods Enzymol. 1995;254:241–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P51 RR000166/RR/NCRR NIH HHS/United States
- AI22646/AI/NIAID NIH HHS/United States
- T32 GM007270/GM/NIGMS NIH HHS/United States
- R01 AI022646/AI/NIAID NIH HHS/United States
- RR00166/RR/NCRR NIH HHS/United States
- 32 GM07270/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases