Increased expression of adenylylcyclase type VI proportionately increases beta-adrenergic receptor-stimulated production of cAMP in neonatal rat cardiac myocytes - PubMed (original) (raw)
Increased expression of adenylylcyclase type VI proportionately increases beta-adrenergic receptor-stimulated production of cAMP in neonatal rat cardiac myocytes
M Gao et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Cellular content of cAMP generated by activation of adenylylcyclase (AC; EC 4.6.1.1) is a key determinant of functional responsiveness in the heart and other tissues. We have tested two hypotheses regarding the relationship between AC content and beta-adrenergic receptor (betaAR)-mediated signal transduction in cardiac myocytes. First, that AC content limits adrenergic signal transduction, and, second, that increased AC, independent of (betaAR) number and G-protein content, yields a proportional increase in betaAR-mediated transmembrane signaling. We used recombinant adenovirus to increase AC isoform VI (ACVI) expression in neonatal cardiac myocytes. Cells that overexpressed ACVI responded to agonist stimulation with marked increases in cAMP production in proportion to protein expressed. In parallel experiments performed on cells transfected with lacZ (control) or ACVI, [3H]forskolin binding, used to assess AC protein expression, was amplified 6-fold, while betaAR-stimulated cAMP production from these cells was increased 7-fold. No changes in betaAR number, or in the heterotrimeric GTP-binding proteins, Galphas or Galphai2, were observed. Previous studies indicate that increased cardiac expression of betaAR or Galphas does not yield proportional increases in transmembrane adrenergic signaling. In contrast, the current data demonstrate that increased ACVI expression provides a proportional increase in beta-adrenergic signal transduction. Our results show that the amount of AC sets a limit on transmembrane beta-adrenergic signaling. We speculate that similar functional responses are possible in other cell types in which AC plays an important physiological role.
Figures
Figure 1
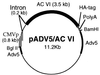
Recombinant adenovirus expressing ACVI. CMVp, cytomegalovirus immediate-early enhancer/promoter region; HA, hemagglutinin protein of influenza virus; PolyA, poly(A) region of bovine growth hormone gene; Adv5, adenovirus 5.
Figure 2
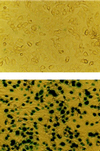
Neonatal cardiac myocytes before (Upper) and after (Lower) lacZ gene transfer. Gene transfer was performed by adding recombinant adenovirus expressing β-galactosidase (1 pfu per cell) and incubating for 20 h. Adenovirus then was removed and cells were maintained for 24 h. The extent of gene transfer was evaluated by 5-bromo-4-chloro-3-indolyl β-
d
-galactoside (X-Gal) staining of cells after gene transfer with lacZ. Lower indicates >95% successful gene transfer. (×80.)
Figure 3
Northern analysis. A murine ACVI cDNA probe was used in Northern blots prepared with RNA extracted from neonatal rat cardiac myocytes after gene transfer using recombinant adenovirus expressing lacZ or ACVI. Transgene mRNA was detectable in control cells only after prolonged exposure, reflecting the low abundance of endogenous ACVI. In contrast, after gene transfer with AdV.ACVI, abundant ACVI mRNA was evident. Equal RNA loading was documented by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) controls (not shown). These data document robust transgene ACVI mRNA expression after gene transfer.
Figure 4
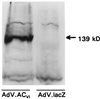
Western analysis. A polyclonal antibody recognizing ACV and ACVI protein (Santa Cruz Biotechnology) was used in Western blots of lysates of neonatal rat cardiac myocytes after gene transfer using recombinant adenovirus expressing ACVI (AdV.ACVI) or lacZ (AdV.lacZ). As expected, transgene protein was undetectable in the control cells. In contrast, after gene transfer with AdV.ACVI, abundant ACVI protein was evident. The amount of total protein loaded was the same in both lanes. These data document robust transgene ACVI protein expression after gene transfer.
Figure 5
(Upper Left) [3H]Forskolin binding. Net 10 μM isoproterenol- and 30 μM GTP[γS]-stimulated [3H]forskolin binding was increased after ACVI gene transfer. These data document that transgene ACVI is present and responsive to Gs–AC interaction. (Lower Left) cAMP production. Cardiac myocytes expressing transgene ACVI have increased adrenergic responsiveness to stimulation not only with 10 μM forskolin, reflecting increased amounts of AC, but also with 10 μM isoproterenol, indicating that newly synthesized AC is functionally coupled and recruitable through βAR stimulation. (Upper Right) Relationship between ACVI content and cAMP production. The graph displays three measures of altered adrenergic signaling ([3H]forskolin binding and isoproterenol- and forskolin-stimulated cAMP production). These data indicate that a proportional increase in AC content and enhanced adrenergic signaling has occurred. Net 30 μM GTP[γS]-stimulated [3H]forskolin binding was increased after ACVI gene transfer. Shown are mean values from three experiments, documenting that transgene ACVI is present and responsive to Gs–AC interaction. In all graphs bars represent mean values from three experiments and error bars denote 1 SD.
Figure 6
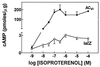
Isoproterenol stimulation. Neonatal rat cardiac myocytes underwent gene transfer using recombinant adenovirus expressing lacZ or ACVI. After gene transfer with ACVI (vs. lacZ), there is an obvious increase in cAMP produced through a wide range of isoproterenol concentrations. The apparent EC50 for isoproterenol-stimulated cAMP production was unchanged.
Figure 7
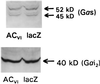
G-protein Western analysis. Polyclonal antibodies recognizing Gαs (Upper) and Gαi2 (Lower) were used in Western blots of lysates of neonatal rat cardiac myocytes after gene transfer using recombinant adenovirus expressing ACVI (AdV.ACVI) or lacZ (AdV.lacZ). Equal amounts of protein were loaded in each lane. Gene transfer had no effect on immunodetectable Gαs or Gαi2 content. These data indicate that substantial expression of ACVI transgene protein does not alter Gαs or Gαi2 content.
Similar articles
- Adenylylcyclase increases responsiveness to catecholamine stimulation in transgenic mice.
Gao MH, Lai NC, Roth DM, Zhou J, Zhu J, Anzai T, Dalton N, Hammond HK. Gao MH, et al. Circulation. 1999 Mar 30;99(12):1618-22. doi: 10.1161/01.cir.99.12.1618. Circulation. 1999. PMID: 10096940 - Effect of overexpressed adenylyl cyclase VI on beta 1- and beta 2-adrenoceptor responses in adult rat ventricular myocytes.
Stark JC, Haydock SF, Foo R, Brown MJ, Harding SE. Stark JC, et al. Br J Pharmacol. 2004 Oct;143(4):465-76. doi: 10.1038/sj.bjp.0705976. Epub 2004 Sep 20. Br J Pharmacol. 2004. PMID: 15381636 Free PMC article. - Cardiac-directed adenylyl cyclase expression improves heart function in murine cardiomyopathy.
Roth DM, Gao MH, Lai NC, Drumm J, Dalton N, Zhou JY, Zhu J, Entrikin D, Hammond HK. Roth DM, et al. Circulation. 1999 Jun 22;99(24):3099-102. doi: 10.1161/01.cir.99.24.3099. Circulation. 1999. PMID: 10377071 - Beta-adrenergic receptor-G protein-adenylyl cyclase signal transduction in the failing heart.
Vatner DE, Asai K, Iwase M, Ishikawa Y, Shannon RP, Homcy CJ, Vatner SF. Vatner DE, et al. Am J Cardiol. 1999 Jun 17;83(12A):80H-85H. doi: 10.1016/s0002-9149(99)00266-0. Am J Cardiol. 1999. PMID: 10750593 Review. - Distinct beta-adrenergic receptor subtype signaling in the heart and their pathophysiological relevance.
Zheng M, Han QD, Xiao RP. Zheng M, et al. Sheng Li Xue Bao. 2004 Feb 25;56(1):1-15. Sheng Li Xue Bao. 2004. PMID: 14985822 Review.
Cited by
- Developmental neurotoxicity elicited by gestational exposure to chlorpyrifos: when is adenylyl cyclase a target?
Meyer A, Seidler FJ, Cousins MM, Slotkin TA. Meyer A, et al. Environ Health Perspect. 2003 Dec;111(16):1871-6. doi: 10.1289/ehp.6468. Environ Health Perspect. 2003. PMID: 14644659 Free PMC article. - Current Landscape of Heart Failure Gene Therapy.
Kieserman JM, Myers VD, Dubey P, Cheung JY, Feldman AM. Kieserman JM, et al. J Am Heart Assoc. 2019 May 21;8(10):e012239. doi: 10.1161/JAHA.119.012239. J Am Heart Assoc. 2019. PMID: 31070089 Free PMC article. Review. No abstract available. - Galphaq reduces cAMP production by decreasing Galphas protein abundance.
Tang T, Gao MH, Miyanohara A, Hammond HK. Tang T, et al. Biochem Biophys Res Commun. 2008 Dec 12;377(2):679-684. doi: 10.1016/j.bbrc.2008.10.054. Epub 2008 Oct 21. Biochem Biophys Res Commun. 2008. PMID: 18948082 Free PMC article. - Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase.
Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Swaney JS, et al. Proc Natl Acad Sci U S A. 2005 Jan 11;102(2):437-42. doi: 10.1073/pnas.0408704102. Epub 2004 Dec 29. Proc Natl Acad Sci U S A. 2005. PMID: 15625103 Free PMC article. - Intracoronary Gene Transfer of Adenylyl Cyclase 6 in Patients With Heart Failure: A Randomized Clinical Trial.
Hammond HK, Penny WF, Traverse JH, Henry TD, Watkins MW, Yancy CW, Sweis RN, Adler ED, Patel AN, Murray DR, Ross RS, Bhargava V, Maisel A, Barnard DD, Lai NC, Dalton ND, Lee ML, Narayan SM, Blanchard DG, Gao MH. Hammond HK, et al. JAMA Cardiol. 2016 May 1;1(2):163-71. doi: 10.1001/jamacardio.2016.0008. JAMA Cardiol. 2016. PMID: 27437887 Free PMC article. Clinical Trial.
References
- Alousi A, Jasper J R, Insel P A, Motulsky H J. FASEB J. 1992;5:2300–2303. - PubMed
- Milano C A, Allen L F, Rockman H A, Dolber P C, McMinn T R, Chien K R, Johnson T D, Bond R A, Lefkowitz R J. Science. 1994;264:582–586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 HL009097/HL/NHLBI NIH HHS/United States
- HL02812-01/HL/NHLBI NIH HHS/United States
- HL09097-01/HL/NHLBI NIH HHS/United States
- T32 HL007444/HL/NHLBI NIH HHS/United States
- 1 P50 HL-53773-01/HL/NHLBI NIH HHS/United States
- P50 HL053773/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources