Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1) - PubMed (original) (raw)
Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1)
M Cheng et al. Proc Natl Acad Sci U S A. 1998.
Abstract
A constitutively active form of mitogen-activated protein kinase kinase (MEK1) was synthesized under control of a zinc-inducible promoter in NIH 3T3 fibroblasts. Zinc treatment of serum-starved cells activated extracellular signal-regulated protein kinases (ERKs) and induced expression of cyclin D1. Newly synthesized cyclin D1 assembled with cyclin-dependent kinase-4 (CDK4) to form holoenzyme complexes that phosphorylated the retinoblastoma protein inefficiently. Activation of the MEK1/ERK pathway neither triggered degradation of the CDK inhibitor kinase inhibitory protein-1 (p27(Kip1)) nor led to activation of cyclin E- and A-dependent CDK2, and such cells did not enter the DNA synthetic (S) phase of the cell division cycle. In contrast, zinc induction of active MEK1 in cells also engineered to ectopically overexpress cyclin D1 and CDK4 subunits generated levels of cyclin D-dependent retinoblastoma protein kinase activity approximating those achieved in cells stimulated by serum. In this setting, p27(Kip1) was mobilized into complexes containing cyclin D1; cyclin E- and A-dependent CDK2 complexes were activated; and serum-starved cells entered S phase. Thus, although the activity of p27(Kip1) normally is canceled through a serum-dependent degradative process, overexpressed cyclin D1-CDK complexes sequestered p27(Kip1) and reduced the effective inhibitory threshold through a stoichiometric mechanism. A fraction of these cells completed S phase and divided, but they were unable to continuously proliferate, indicating that other serum-responsive factors ultimately became rate limiting for cell cycle progression. Therefore, the MEK/ERK pathway not only acts transcriptionally to induce the cyclin D1 gene but functions posttranslationally to regulate cyclin D1 assembly with CDK4 and to thereby help cancel p27(Kip1)-mediated inhibition.
Figures
Figure 1
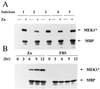
Inducible expression of MEK1* in NIH 3T3 fibroblasts leads to activation of ERK1. (A) Five subclones expressing inducible MEK1* were serum-starved and restimulated with ZnS04 for 12 hr. MEK1* was detected by immunoblotting with antibody 9E10 to the myc tag, and activation of ERK1 myelin basic protein kinase activity was evaluated in immune complexes. (B) Starved cells from subclone 3 were restimulated with ZnSO4 or 10% FBS for the indicated times and assayed for MEK1* expression and ERK1 kinase activity as in A.
Figure 2
MEK1* induces cyclin D1 synthesis and assembly with CDK4. Serum-starved cells (subclone 3) harboring inducible MEK1* were stimulated with ZnSO4 or FBS and assayed at the indicated times thereafter (hr) for cyclin D1 and CDK4 expression (A), assembly (B), and RB kinase activity (C).
Figure 3
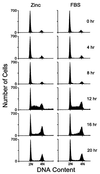
Molecular correlates of S phase entry. (A) Cells from five individual subclones expressing inducible MEK1* were serum-starved and restimulated with ZnSO4 or FBS for 16 hr, and the S phase fraction was determined by using flow cytometry to estimate the DNA content of propidium iodide-stained nuclei. (B) Serum-starved cells (subclone 3) stimulated with ZnSO4 or FBS were assayed at 4-hr intervals for DNA content (Upper). Cyclin A, p21Cip1, and p27Kip1 levels were determined by immunoblotting. Precipitates recovered with antibodies to cyclin E and CDK2 were assayed for histone H1 kinase activity.
Figure 4
Expression of MEK1* in cells overexpressing cyclin D1 and CDK4. Serum-starved cells from three subclones coexpressing cyclin D1, CDK4, and inducible MEK1* were stimulated with ZnSO4 or FBS for 12 hr. Cell lysates were assayed for expression of MEK1*, cyclin D1, and CDK4 by immunoblotting (A), cyclin D1-CDK4 complex formation by immunoprecipitation with CDK4 antibody followed by immunoblotting with cyclin D1 antibody (B), and CDK4-associated RB kinase activity (C).
Figure 5
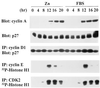
Overexpression of cyclin D1, CDK4, and MEK1* induces cyclin A synthesis and CDK2 activation, but not p27Kip1 degradation. Cells (subclone 2) ectopically expressing cyclin D1, CDK4, and inducible MEK1* were starved and restimulated with ZnSO4 or FBS. Cells harvested at intervals thereafter (hr) were assayed for cyclin A and p27Kip1 expression by immunoblotting (top two panels), for cyclin D1-associated p27Kip1 by immunoprecipitation with cyclin D1 followed by immunoblotting with anti-p27Kip1 (middle panel), and for cyclin E- and CDK2-associated histone H1 kinase activity (bottom two panels). More than 85% of p27Kip1 coprecipitated with cyclin D1.
Figure 6
Ectopic expression of cyclin D1, CDK4, and MEK1* induces S phase entry. Serum-starved cells (subclone 2) ectopically expressing cyclin D1, CDK4, and inducible MEK1* were stimulated with ZnSO4 or FBS, harvested at indicated times thereafter, and assayed for DNA content by flow cytometry.
References
- Sherr C J, Roberts J M. Genes Dev. 1995;9:1149–1163. - PubMed
- Sherr C J. Cell. 1994;79:551–555. - PubMed
- Morgan D O. Nature (London) 1995;374:131–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous