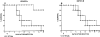Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response - PubMed (original) (raw)
Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response
K Goldsmith et al. J Exp Med. 1998.
Abstract
The herpes simplex virus (HSV) infected cell protein (ICP)47 blocks CD8+ T cell recognition of infected cells by inhibiting the transporter associated with antigen presentation (TAP). In vivo, HSV-1 replicates in two distinct tissues: in epithelial mucosa or epidermis, where the virus enters sensory neurons; and in the peripheral and central nervous system, where acute and subsequently latent infections occur. Here, we show that an HSV-1 ICP47- mutant is less neurovirulent than wild-type HSV-1 in mice, but replicates normally in epithelial tissues. The reduced neurovirulence of the ICP47- mutant was due to a protective CD8+ T cell response. When compared with wild-type virus, the ICP47- mutant expressed reduced neurovirulence in immunologically normal mice, and T cell-deficient nude mice after reconstitution with CD8+ T cells. However, the ICP47- mutant exhibited normal neurovirulence in mice that were acutely depleted of CD8+ T cells, and in nude mice that were not reconstituted, or were reconstituted with CD4+ T cells. In contrast, CD8+ T cell depletion did not increase the neurovirulence of an unrelated, attenuated HSV-1 glycoprotein (g)E- mutant. ICP47 is the first viral protein shown to influence neurovirulence by inhibiting CD8+ T cell protection.
Figures
Figure 1
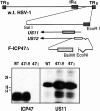
Generation of an HSV-1 ICP47 deletion mutant. The genome of HSV-1 is depicted in the upper panel of the figure. A 100- nucleotide deletion in the HSV US12 gene, which encodes ICP47, was created. The deletion removes the ICP47 start codon and two downstream ATG codons, but does not affect the promoter of the adjacent US11 gene. Cells were cotransfected with a plasmid containing the deletion, and viral DNA derived from HSV-1 (strain F). Virus progeny were screened for the mutation by performing PCR on samples of viral DNA. A virus, denoted F-ICP47Δ, contained the deletion in the US12 gene. A rescued derivative of F-ICP47Δ, denoted F-ICP47ΔR, was produced by cotransfecting cells with F-ICP47Δ DNA and a plasmid containing the wild-type US12 gene. In the lower panel, cells were infected with wild-type HSV-1, F-ICP47Δ, or F-ICP47ΔR, then radiolabeled with [35S]methionine. ICP47 was immunoprecipitated from cell extracts using a rabbit anti-ICP47 serum, and US11 protein was immunoprecipitated using an anti-US11 serum.
Figure 2
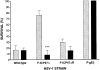
ICP47 enhances neurovirulence in mice. In 3 experiments, groups of 10 female A/J mice were depleted of CD8+ T cells by injection of anti-CD8 mAb (solid bars), or were mock depleted by injection of control mAb (gray bars). The mice received uniocular corneal infections with 105 PFU of wild-type HSV-1 F strain, F-ICP47Δ, or F-ICP47ΔR, or F-gEβ. The survival rate was determined after a 30-d observation period, although all deaths occurred 12–14 d after infection. The *** indicates that the survival rate was significantly increased (P < 0.001) in immunologically normal mice infected with the F-ICP47Δ deletion mutant when compared with one of the following: CD8+ T cell–depleted mice infected with F-ICP47Δ; immunologically normal mice infected with wild-type F; or immunologically normal mice infected with F-ICP47ΔR.
Figure 3
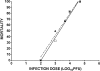
ICP47 does not enhance neurovirulence in T cell– deficient mice. Groups of six BALB/c athymic nude mice received corneal infections with various doses of F-ICP47Δ (▴) or F-ICP47ΔR (□). The mortality rate was observed over a 30-d observation period. The symbols represent the percentage of mortality at each infectious dose. The linear regression lines are shown for each data set. There is no significant difference in the slopes (P = 0.6604) or the elevations and intercepts (P = 0.3738) of the two lines.
Figure 4
Adoptive transfer of CD8+, but not CD4+, T cells inhibits neurovirulence of the ICP47− mutant in nude mice. Groups of five BALB/c athymic nude mice received corneal infections with 105 PFU of F-ICP47Δ HSV-1 (A), or F-ICP47ΔR HSV-1 (B). 6 d later, the control group (□) received no cells, or the mice received 1.5 × 107 lymph node cells from HSV-1– infected euthymic BALB/c mice that were highly enriched for CD4+ (▵) CD8+ (○) T lymphocytes. Survival of the recipient mice was followed through a 30-d observation period. Adoptive transfer of CD8+ cells conferred a significant survival advantage for mice infected with the F-ICP47Δ strain (P = 0.0186), but not for mice infected with the F-ICP47ΔR strain (P = 0.3962). Adoptive transfer of CD4+ cells did not significantly alter (P >0.05) survival of mice infected with either virus.
Similar articles
- Inhibition of major histocompatibility complex class I antigen presentation in pig and primate cells by herpes simplex virus type 1 and 2 ICP47.
Jugovic P, Hill AM, Tomazin R, Ploegh H, Johnson DC. Jugovic P, et al. J Virol. 1998 Jun;72(6):5076-84. doi: 10.1128/JVI.72.6.5076-5084.1998. J Virol. 1998. PMID: 9573278 Free PMC article. - Suppression of CD80 Expression by ICP22 Affects Herpes Simplex Virus Type 1 Replication and CD8+IFN-γ+ Infiltrates in the Eyes of Infected Mice but Not Latency Reactivation.
Matundan HH, Wang S, Jaggi U, Yu J, Ghiasi H. Matundan HH, et al. J Virol. 2021 Sep 9;95(19):e0103621. doi: 10.1128/JVI.01036-21. Epub 2021 Sep 9. J Virol. 2021. PMID: 34287036 Free PMC article. - CXCL10/CXCR3-Dependent Mobilization of Herpes Simplex Virus-Specific CD8+ TEM and CD8+ TRM Cells within Infected Tissues Allows Efficient Protection against Recurrent Herpesvirus Infection and Disease.
Srivastava R, Khan AA, Chilukuri S, Syed SA, Tran TT, Furness J, Bahraoui E, BenMohamed L. Srivastava R, et al. J Virol. 2017 Jun 26;91(14):e00278-17. doi: 10.1128/JVI.00278-17. Print 2017 Jul 15. J Virol. 2017. PMID: 28468883 Free PMC article. - Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo.
Leib DA, Harrison TE, Laslo KM, Machalek MA, Moorman NJ, Virgin HW. Leib DA, et al. J Exp Med. 1999 Feb 15;189(4):663-72. doi: 10.1084/jem.189.4.663. J Exp Med. 1999. PMID: 9989981 Free PMC article. - The active domain of the herpes simplex virus protein ICP47: a potent inhibitor of the transporter associated with antigen processing.
Neumann L, Kraas W, Uebel S, Jung G, Tampé R. Neumann L, et al. J Mol Biol. 1997 Oct 3;272(4):484-92. doi: 10.1006/jmbi.1997.1282. J Mol Biol. 1997. PMID: 9325106
Cited by
- Tumour-intrinsic resistance to immune checkpoint blockade.
Kalbasi A, Ribas A. Kalbasi A, et al. Nat Rev Immunol. 2020 Jan;20(1):25-39. doi: 10.1038/s41577-019-0218-4. Epub 2019 Sep 30. Nat Rev Immunol. 2020. PMID: 31570880 Free PMC article. Review. - Requirement of an integrated immune response for successful neuroattenuated HSV-1 therapy in an intracranial metastatic melanoma model.
Miller CG, Fraser NW. Miller CG, et al. Mol Ther. 2003 Jun;7(6):741-7. doi: 10.1016/s1525-0016(03)00120-5. Mol Ther. 2003. PMID: 12788647 Free PMC article. - Current and Emerging Therapies for Ocular Herpes Simplex Virus Type-1 Infections.
Koganti R, Yadavalli T, Shukla D. Koganti R, et al. Microorganisms. 2019 Oct 10;7(10):429. doi: 10.3390/microorganisms7100429. Microorganisms. 2019. PMID: 31658632 Free PMC article. Review. - Herpes simplex virus type 1 induces CD83 degradation in mature dendritic cells with immediate-early kinetics via the cellular proteasome.
Kummer M, Turza NM, Muhl-Zurbes P, Lechmann M, Boutell C, Coffin RS, Everett RD, Steinkasserer A, Prechtel AT. Kummer M, et al. J Virol. 2007 Jun;81(12):6326-38. doi: 10.1128/JVI.02327-06. Epub 2007 Apr 11. J Virol. 2007. PMID: 17428858 Free PMC article. - The expression and function of HSV ICP47 and its promoter in mice.
Velusamy T, Singh N, Croft S, Smith S, Tscharke DC. Velusamy T, et al. J Virol. 2023 Nov 30;97(11):e0110723. doi: 10.1128/jvi.01107-23. Epub 2023 Oct 30. J Virol. 2023. PMID: 37902400 Free PMC article.
References
- Früh K, Ahn K, Djaballah H, Sempe P, van Endert PM, Tampe R, Peterson PA, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. - PubMed
- Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off TAP to evade host immunity. Nature. 1995;375:411–415. - PubMed
- Johnson, D.C., and A.B. Hill. 1998. Herpesviruses and immune evasion. Curr. Top. Microbiol. Immunol. In press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY-11245/EY/NEI NIH HHS/United States
- P30 EY001792/EY/NEI NIH HHS/United States
- EY-01792/EY/NEI NIH HHS/United States
- R01 EY005945/EY/NEI NIH HHS/United States
- EY-05945/EY/NEI NIH HHS/United States
- R01 EY011245/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous