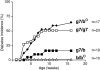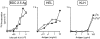Major histocompatibility complex class II molecules can protect from diabetes by positively selecting T cells with additional specificities - PubMed (original) (raw)
Major histocompatibility complex class II molecules can protect from diabetes by positively selecting T cells with additional specificities
F Lühder et al. J Exp Med. 1998.
Abstract
Insulin-dependent diabetes is heavily influenced by genes encoded within the major histocompatibility complex (MHC), positively by some class II alleles and negatively by others. We have explored the mechanism of MHC class II-mediated protection from diabetes using a mouse model carrying the rearranged T cell receptor (TCR) transgenes from a diabetogenic T cell clone derived from a nonobese diabetic mouse. BDC2.5 TCR transgenics with C57Bl/6 background genes and two doses of the H-2(g7) allele exhibited strong insulitis at approximately 3 wk of age and most developed diabetes a few weeks later. When one of the H-2(g7) alleles was replaced by H-2(b), insulitis was still severe and only slightly delayed, but diabetes was markedly inhibited in both its penetrance and time of onset. The protective effect was mediated by the Abetab gene, and did not merely reflect haplozygosity of the Abetag7 gene. The only differences we observed in the T cell compartments of g7/g7 and g7/b mice were a decrease in CD4(+) cells displaying the transgene-encoded TCR and an increase in cells expressing endogenously encoded TCR alpha-chains. When the synthesis of endogenously encoded alpha-chains was prevented, the g7/b animals were no longer protected from diabetes. g7/b mice did not have a general defect in the production of Ag7-restricted T cells, and antigen-presenting cells from g7/b animals were as effective as those from g7/g7 mice in stimulating Ag7-restricted T cell hybridomas. These results argue against mechanisms of protection involving clonal deletion or anergization of diabetogenic T cells, or one depending on capture of potentially pathogenic Ag7-restricted epitopes by Ab molecules. Rather, they support a mechanism based on MHC class II-mediated positive selection of T cells expressing additional specificities.
Figures
Figure 1
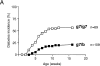
The H-2b complex, but not the E molecule, protects BDC2.5/C57 tgs from diabetes. (A) BDC2.5/C57 g7/g7 and g7/b littermates were followed for diabetes; the percentage of diabetic mice over time is indicated. The data are pooled from several independent cohorts. (B) BDC2.5/ C57 g7/g7 and g7/b littermates, carrying or not carrying the Eα16 transgene, were monitored for diabetes.
Figure 1
The H-2b complex, but not the E molecule, protects BDC2.5/C57 tgs from diabetes. (A) BDC2.5/C57 g7/g7 and g7/b littermates were followed for diabetes; the percentage of diabetic mice over time is indicated. The data are pooled from several independent cohorts. (B) BDC2.5/ C57 g7/g7 and g7/b littermates, carrying or not carrying the Eα16 transgene, were monitored for diabetes.
Figure 2
The onset of insulitis is delayed in g7/b BDC2.5 tgs. BDC2.5/C57 transgenic mice (g7/g7 or g7/b haplotype at the MHC) were killed on days 12, 18, 24, or 30, and the percentage of pancreatic islets showing either periinsulitis or insulitis was quantitated. Each bar represents an individual mouse. The white portion of the bars indicates periinsulitis, the black portion insulitis.
Figure 3
The protective effect of the H-2b complex can be attributed to Ab molecules. BDC2.5/C57 transgenic mice were bred with g7, b, or b° haplotypes at the MHC. The latter is a mutant H-2b complex carrying a null mutation at the Aβ locus (38). Littermates from these crosses were followed for diabetes.
Figure 4
In g7/b BDC2.5 TCR tg mice, there are fewer CD4+ T cells and more cells that express endogenously encoded TCR-α chains. (A) Total numbers of thymocytes in g7/g7 and g7/b littermates from 10 different experiments; each dot represents an individual mouse; the average values are shown (g7/g7: 159.5 ± 78.3 millions, g7/b: 160.4 ± 75.4 millions). (B) Representative CD4/CD8 cytofluorimetric profiles; the percentage of CD4+8− cells is indicated. (C) Numbers of CD4+8− cells; the average values are shown (g7/g7: 24.8 ± 11.7 millions, g7/b: 10.3 ± 6.2 millions, P <0.003). (D) Representative histogram of CD3 expression on total thymocytes. (E) Representative CD4/CD8 profiles of mesenteric lymph node lymphocytes, gated on CD3-positive cells. The percentages of CD4+ and CD8+ cells are indicated. (F) Percentage of CD4+ cells for g7/g7 and g7/b littermates; each dot represents an individual mouse; the average values are shown (g7/g7: 20 ± 8.9 millions, g7/b: 11.6 ± 4.1 millions, P <0.005). The total number of lymph node cells did not differ significantly in the different mice. (G) TCR-αβ/Vα2 profiles of CD4+ T cells; the two gates delineate the Vα2hi and Vα2int populations; in normal C57Bl/6 mice taken as a reference, the vast majority (80–90%) of Vα2-positive CD4+ cells fell within this Vα2hi gate. (H) Black bars represent proportion of cells expressing an endogeneous Vα chain (relative to C57Bl/6); white bars represent proportion of cells expressing low levels of an endogeneous Vα chain (most likely in conjunction with the tg Vα), also relative to C57Bl/6. Each bar represents an individual mouse.
Figure 4
In g7/b BDC2.5 TCR tg mice, there are fewer CD4+ T cells and more cells that express endogenously encoded TCR-α chains. (A) Total numbers of thymocytes in g7/g7 and g7/b littermates from 10 different experiments; each dot represents an individual mouse; the average values are shown (g7/g7: 159.5 ± 78.3 millions, g7/b: 160.4 ± 75.4 millions). (B) Representative CD4/CD8 cytofluorimetric profiles; the percentage of CD4+8− cells is indicated. (C) Numbers of CD4+8− cells; the average values are shown (g7/g7: 24.8 ± 11.7 millions, g7/b: 10.3 ± 6.2 millions, P <0.003). (D) Representative histogram of CD3 expression on total thymocytes. (E) Representative CD4/CD8 profiles of mesenteric lymph node lymphocytes, gated on CD3-positive cells. The percentages of CD4+ and CD8+ cells are indicated. (F) Percentage of CD4+ cells for g7/g7 and g7/b littermates; each dot represents an individual mouse; the average values are shown (g7/g7: 20 ± 8.9 millions, g7/b: 11.6 ± 4.1 millions, P <0.005). The total number of lymph node cells did not differ significantly in the different mice. (G) TCR-αβ/Vα2 profiles of CD4+ T cells; the two gates delineate the Vα2hi and Vα2int populations; in normal C57Bl/6 mice taken as a reference, the vast majority (80–90%) of Vα2-positive CD4+ cells fell within this Vα2hi gate. (H) Black bars represent proportion of cells expressing an endogeneous Vα chain (relative to C57Bl/6); white bars represent proportion of cells expressing low levels of an endogeneous Vα chain (most likely in conjunction with the tg Vα), also relative to C57Bl/6. Each bar represents an individual mouse.
Figure 5
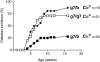
Introduction of the TCR-α° mutation restores high diabetes incidence in g7/b mice. BDC2.5/C57 mice (g7/g7 or g7/b haplotypes at the MHC) were bred with the TCR-α–null mutation. Cα° homozygotes or Cα+ littermates were followed for diabetes (since there was no difference between homozygous and heterozygous wild-type animals, the data were pooled, indicated as Cα+). The diabetes incidence for Cα° g7/g7 mice did not differ from that of Cα+ littermates and is therefore not shown here for clarity.
Figure 6
APCs from both g7/g7 and g7/b mice present antigen efficiently to Ag7-restricted T cell hybridomas. The BDC2.5 hybridoma or g7-restricted hybridomas specific for HEL or KLH epitopes [HEL(g7) 4B12 and KLH(g7)5D2, respectively] were challenged with APCs from non-tg g7/g7 or g7/b animals. The antigen titration curves were made with islet cells or with HEL or KLH protein, and IL-2 production was read-out as [3H]thymidine incorporation by the IL-2–dependent CTLL cell line.
Similar articles
- A mechanism for the major histocompatibility complex-linked resistance to autoimmunity.
Schmidt D, Verdaguer J, Averill N, Santamaria P. Schmidt D, et al. J Exp Med. 1997 Oct 6;186(7):1059-75. doi: 10.1084/jem.186.7.1059. J Exp Med. 1997. PMID: 9314555 Free PMC article. - MHC class II molecules play a role in the selection of autoreactive class I-restricted CD8 T cells that are essential contributors to type 1 diabetes development in nonobese diabetic mice.
Serreze DV, Holl TM, Marron MP, Graser RT, Johnson EA, Choisy-Rossi C, Slattery RM, Lieberman SM, DiLorenzo TP. Serreze DV, et al. J Immunol. 2004 Jan 15;172(2):871-9. doi: 10.4049/jimmunol.172.2.871. J Immunol. 2004. PMID: 14707058 - Antigen presentation in the autoimmune diabetes of the NOD mouse.
Unanue ER. Unanue ER. Annu Rev Immunol. 2014;32:579-608. doi: 10.1146/annurev-immunol-032712-095941. Epub 2014 Feb 5. Annu Rev Immunol. 2014. PMID: 24499272 Review. - T-cell receptor transgenic (TCR-Tg) mice from two diabetogenic CD4+ islet-antigen-specific T-cell clones.
Haskins K. Haskins K. J Autoimmun. 2004 Mar;22(2):107-9. doi: 10.1016/j.jaut.2003.10.006. J Autoimmun. 2004. PMID: 14987737 Review. No abstract available.
Cited by
- Promiscuous binding of proinsulin peptides to Type 1 diabetes-permissive and -protective HLA class II molecules.
Astill TP, Ellis RJ, Arif S, Tree TI, Peakman M. Astill TP, et al. Diabetologia. 2003 Apr;46(4):496-503. doi: 10.1007/s00125-003-1070-3. Epub 2003 Apr 9. Diabetologia. 2003. PMID: 12684749 - Induction of mixed chimerism with MHC-mismatched but not matched bone marrow transplants results in thymic deletion of host-type autoreactive T-cells in NOD mice.
Racine J, Wang M, Zhang C, Lin CL, Liu H, Todorov I, Atkinson M, Zeng D. Racine J, et al. Diabetes. 2011 Feb;60(2):555-64. doi: 10.2337/db10-0827. Diabetes. 2011. PMID: 21270266 Free PMC article. - Modifier loci condition autoimmunity provoked by Aire deficiency.
Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Jiang W, et al. J Exp Med. 2005 Sep 19;202(6):805-15. doi: 10.1084/jem.20050693. J Exp Med. 2005. PMID: 16172259 Free PMC article. - The role of t/b lymphocyte collaboration in the regulation of autoimmune and alloimmune responses.
Noorchashm H, Greeley SA, Naji A. Noorchashm H, et al. Immunol Res. 2003;27(2-3):443-50. doi: 10.1385/IR:27:2-3:443. Immunol Res. 2003. PMID: 12857987 Review. - Dissecting autoimmune diabetes through genetic manipulation of non-obese diabetic mice.
Yang Y, Santamaria P. Yang Y, et al. Diabetologia. 2003 Nov;46(11):1447-64. doi: 10.1007/s00125-003-1218-1. Epub 2003 Oct 28. Diabetologia. 2003. PMID: 14586501 Review.
References
- Bach JF. Insulin-dependent diabetes mellitus as an autoimmune disease. Endocr Rev. 1994;15:516–542. - PubMed
- Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. - PubMed
- Wicker LS, Todd JA, Peterson LB. Genetic control of autoimmune diabetes in the NOD mouse. Annu Rev Immunol. 1995;13:179–200. - PubMed
- Julier C, Hashimoto L, Lathrop GM. Genetics of insulin-dependent diabetes mellitus. Curr Opin Genet Dev. 1996;6:354–360. - PubMed
- Hattori M, Buse JB, Jackson RA, Glimcher L, Dorf ME, Minami M, Makino S, Moriwaki K, Kuzuya H, Imura H, et al. The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science. 1986;231:733–735. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials