S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe - PubMed (original) (raw)
S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe
H D Lindsay et al. Genes Dev. 1998.
Abstract
Checkpoints that respond to DNA structure changes were originally defined by the inability of yeast mutants to prevent mitosis following DNA damage or S-phase arrest. Genetic analysis has subsequently identified subpathways of the DNA structure checkpoints, including the reversible arrest of DNA synthesis. Here, we show that the Cds1 kinase is required to slow S phase in the presence of DNA-damaging agents. Cds1 is phosphorylated and activated by S-phase arrest and activated by DNA damage during S phase, but not during G1 or G2. Activation of Cds1 during S phase is dependent on all six checkpoint Rad proteins, and Cds1 interacts both genetically and physically with Rad26. Unlike its Saccharomyces cerevisiae counterpart Rad53, Cds1 is not required for the mitotic arrest checkpoints and, thus, defines an S-phase specific subpathway of the checkpoint response. We propose a model for the DNA structure checkpoints that offers a new perspective on the function of the DNA structure checkpoint proteins. This model suggests that an intrinsic mechanism linking S phase and mitosis may function independently of the known checkpoint proteins.
Figures
Figure 1
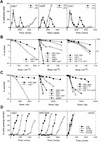
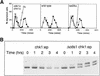
Checkpoint and survival analysis of cds1 mutants. All designations indicate null alleles, with the exception of r26.T12, which designates the rad26.T12 allele. (A) cds1 null mutant cells display mitotic arrest following inhibition of S phase with HU. Synchronous cultures of G2 cells were generated on lactose gradients (Barbet and Carr 1993) and divided into two samples, one of which was incubated in 10 m
m
HU. Septation index and DAPI staining were used to follow progress through the cell cycle. cds1 mutants arrested mitosis for over 6 hr following S-phase arrest. In contrast, a representative of the checkpoint rad group of mutants, rad3, entered mitosis without completing S phase. (B,C) Radiation survival of logarithmically growing cells show that cds1 null mutant cells are sensitive to damaging agents and show increased sensitivity with chk1, but not rad26.T12. (D) cds1 cells, in contrast to checkpoint-defective (rad26) cells have a normal DNA-damage checkpoint at mitosis. Synchronous cultures of G2 cells were generated on lactose gradients and dividend into four samples. One sample was not irradiated, whereas one each was irradiated with 50, 250, or 500 Gy of ionizing radiation.
Figure 2
cds1 is a multicopy suppresser of rad26.T12 mutant cells. (A) rad26 null and rad26.T12 mutant cells were transformed with rad26 genomic DNA, cds1 genomic DNA, or an empty vector control (pAL). Cells were serially diluted (from 107 cells/ml to 104 cells/ml), spotted onto plates and irradiated at the doses indicated. Cells were also spotted onto plates containing a range of HU concentrations. (B) rad26 null cells were transformed with the rad26 genomic clone, the empty vector (pAL) or rad26 constructs containing carboxy-terminal deletions. Numbers correspond to the length of the constructs (amino acids). Full-length Rad26 is 614 amino acids. Cells were diluted to the concentrations shown and either spotted onto plates and irradiated at the doses indicated or spotted onto plates containing different concentrations of HU. (C) Cds1 immunoprecipitates with Rad26 in S. pombe extracts. Wild-type S. pombe was transformed with REP41 (leucine selection) and REP42 (uracil selection) plasmids containing cds1 and rad26 cDNA tagged with either HA or Myc epitopes. Immunoprecipitation was carried out on extracts from logarithmically growing cells, after 18 hr growth without thiamine. Immunocomplexes were processed for Western blotting with anti-Myc antibody. Rad26 associates with itself (lane 3) and with Cds1 (lanes 7,8). The empty vector (lanes 1,2,4,5) was used as control. We estimate overexpression at ∼50× of wild-type protein levels. Comparing the amount of Cds1 precipitated with Rad26 to total protein, we estimate ∼3%–5% recovery. (D) Immunoprecipitations were repeated by use of proteins expressed in rabbit reticulocyte lysate. In this case, Rad26 was seen to associate with itself, but not with Cds1, suggesting bridging proteins or posttranslational modification are required for the Rad26–Cds1 interaction. This would be consistent with levels of recovery.
Figure 2
cds1 is a multicopy suppresser of rad26.T12 mutant cells. (A) rad26 null and rad26.T12 mutant cells were transformed with rad26 genomic DNA, cds1 genomic DNA, or an empty vector control (pAL). Cells were serially diluted (from 107 cells/ml to 104 cells/ml), spotted onto plates and irradiated at the doses indicated. Cells were also spotted onto plates containing a range of HU concentrations. (B) rad26 null cells were transformed with the rad26 genomic clone, the empty vector (pAL) or rad26 constructs containing carboxy-terminal deletions. Numbers correspond to the length of the constructs (amino acids). Full-length Rad26 is 614 amino acids. Cells were diluted to the concentrations shown and either spotted onto plates and irradiated at the doses indicated or spotted onto plates containing different concentrations of HU. (C) Cds1 immunoprecipitates with Rad26 in S. pombe extracts. Wild-type S. pombe was transformed with REP41 (leucine selection) and REP42 (uracil selection) plasmids containing cds1 and rad26 cDNA tagged with either HA or Myc epitopes. Immunoprecipitation was carried out on extracts from logarithmically growing cells, after 18 hr growth without thiamine. Immunocomplexes were processed for Western blotting with anti-Myc antibody. Rad26 associates with itself (lane 3) and with Cds1 (lanes 7,8). The empty vector (lanes 1,2,4,5) was used as control. We estimate overexpression at ∼50× of wild-type protein levels. Comparing the amount of Cds1 precipitated with Rad26 to total protein, we estimate ∼3%–5% recovery. (D) Immunoprecipitations were repeated by use of proteins expressed in rabbit reticulocyte lysate. In this case, Rad26 was seen to associate with itself, but not with Cds1, suggesting bridging proteins or posttranslational modification are required for the Rad26–Cds1 interaction. This would be consistent with levels of recovery.
Figure 3
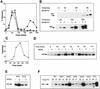
Cds1 is phosphorylated during S-phase arrest. (A) (Left) Immunoprecipitation (top) and kinase activity (bottom) from wild-type and Cds1 null cells by use of affinity-purified Cds1 sera or preimmune sera identifies Cds1 as a single band migrating at 52 kD. In extracts from cells treated with HU, Cds1 undergoes an apparent increase in molecular weight (second lane). Kinase activity against MBP in the IPs correlates with the presence of Cds1 and increases in cells treated with HU. To show the specificity of the kinase activity to Cds1, wild-type or kinase-dead Cds1 protein were expressed in cds1 null cells, immunoprecipitated (top, right) and assayed for kinase activity (bottom, right). (B) The Cds1 mobility shift can be reversed by phosphatase treatment. Ten and twenty units of CIAP restored the mobility of Cds1 from HU-treated cells to that of Cds1 from untreated cells. Twenty units of heat-treated CIAP does not affect Cds1 mobility. (C) Cds1 kinase can be activated by DNA damage. Asynchronous cultures of wild-type and chk1 null cells were subjected to 500 Gy of ionizing radiation. Cell extracts were made at time points following irradiation and assayed for Cds1 kinase activity. Wild-type cells showed a small but reproducible increase in Cds1 kinase activity. chk1 cells showed a greater increase in Cds1 kinase activity. (D,E) Cds1 phosphorylation (D) and activation (E) following HU treatment is dependent on the function of the checkpoint Rad proteins. Cds1 phosphorylation is not seen in immunoprecipitates from rad1, rad3, rad9, rad17, rad26, or hus1 null mutants but is evident in wild-type (WT) and chk1 mutants. IP-kinase activity is much reduced or absent in the checkpoint rad null strains when compared with wild-type and chk1 null cells.
Figure 3
Cds1 is phosphorylated during S-phase arrest. (A) (Left) Immunoprecipitation (top) and kinase activity (bottom) from wild-type and Cds1 null cells by use of affinity-purified Cds1 sera or preimmune sera identifies Cds1 as a single band migrating at 52 kD. In extracts from cells treated with HU, Cds1 undergoes an apparent increase in molecular weight (second lane). Kinase activity against MBP in the IPs correlates with the presence of Cds1 and increases in cells treated with HU. To show the specificity of the kinase activity to Cds1, wild-type or kinase-dead Cds1 protein were expressed in cds1 null cells, immunoprecipitated (top, right) and assayed for kinase activity (bottom, right). (B) The Cds1 mobility shift can be reversed by phosphatase treatment. Ten and twenty units of CIAP restored the mobility of Cds1 from HU-treated cells to that of Cds1 from untreated cells. Twenty units of heat-treated CIAP does not affect Cds1 mobility. (C) Cds1 kinase can be activated by DNA damage. Asynchronous cultures of wild-type and chk1 null cells were subjected to 500 Gy of ionizing radiation. Cell extracts were made at time points following irradiation and assayed for Cds1 kinase activity. Wild-type cells showed a small but reproducible increase in Cds1 kinase activity. chk1 cells showed a greater increase in Cds1 kinase activity. (D,E) Cds1 phosphorylation (D) and activation (E) following HU treatment is dependent on the function of the checkpoint Rad proteins. Cds1 phosphorylation is not seen in immunoprecipitates from rad1, rad3, rad9, rad17, rad26, or hus1 null mutants but is evident in wild-type (WT) and chk1 mutants. IP-kinase activity is much reduced or absent in the checkpoint rad null strains when compared with wild-type and chk1 null cells.
Figure 4
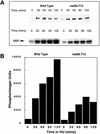
Activation of Cds1 kinase is decreased in rad26.T12 mutant cells. (A) Logarithmically growing wild-type and rad26.T12 cells were treated with 20 m
m
HU and Cds1 mobility (top) and kinase activity (bottom) were assayed from cell extracts prepared at 30 min intervals. (B) The activation of Cds1 kinase is reduced in rad26.T12 cells in response to HU when quantified by PhosphoImager analysis of the autoradiograph shown in A.
Figure 5
Activation of Cds1 kinase is S-phase dependent. (A) Septation of wild-type cells synchronized in G2 by elutriation. The culture was divided into three and incubated at 30°. HU was added (20 m
m
) at either t = 0 (□) or t = 60 (•) min; control (▪). The cell-cycle stage of the culture was followed by septation index by Calcofluor and DAPI staining. Untreated cells show two peaks in septation. The second peak is missing in the HU-treated culture indicating the activation of the replication checkpoint. (B) Samples were taken from all three cultures and processed for Cds1 kinase assay. Cds1 kinase does not activate during G2 despite the presence of 20 m
m
HU (30+, 60+). Cds1 kinase activity accumulates after 90 min when the majority of the cells have entered or are entering S phase. (C) Septation index of wild-type cells synchronized in G2 by elutriation (30°C). (D) Cds1 kinase activity of irradiated or unirradiated cells removed at the times indicated and treated with 250 Gy of radiation. Cell extracts were prepared after 40 min of recovery incubation at 30°C. Cells irradiated in G2 at t = 0 do not activate Cds1 kinase after irradiation, whereas cells entering S phase after irradiation develop significant activity. Consistent with the S-phase dependency of the kinase activation cells irradiated late in S phase/G2 (t = 90,110) fail to generate significant kinase activity compared with the unirradiated control. (E) cdc10.V50 cells irradiated at the permissive temperature (26°C) show activation of Cds1 kinase whereas cells irradiated and held at the restrictive temperature (37°C) do not, thus showing that Cds1 kinase is not activated by DNA damage during G1 phase. (F) Activation of Cds1 by temperature shift in various cdc mutants defective in DNA synthesis. Logarithmic cultures of wild-type, cdc6, cdc17, cdc20, cdc21, cdc22, and _pol_α cells grown at 27°C were split into two samples, one was incubated for a further 2 hr, 30 min at 27°C, whereas the other was incubated at 37°C for 2 hr, 30 min before extracts were prepared and tested for IP kinase activity.
Figure 6
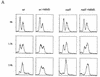
The S-phase progression checkpoint requires rad3, rad26, and cds1. (A) rad+ cells (_h_− cyr1 sxa2) and rad3 mutant cells (h_−_cyr1 sxa2 rad3) were synchronized in G1 by centrifugal elutriation (collecting early G2 cells) followed by exposure to P factor. Cells were released from P factor-induced G1 arrest in either the presence or absence of 0.033% MMS and S-phase progression followed by FACS. Attempts to synchronize other checkpoint mutants this way were complicated by the morphological changes associated with the cyr1 sxa2 background. (B) Wild-type, rad3, cds1, chk1, rad26, and rad26-T12 cells were synchronized in G1 by nitrogen starvation, released into the cell cycle in the presence or absence of 0.033% MMS and followed by FACS. As before, rad3 cells are unable to slow S phase in response to DNA damage. Similarly, cds1 and rad26 are required for this checkpoint, whereas chk1 is not. Cells carrying the rad26.T12 mutation also have a defect in this checkpoint.
Figure 6
The S-phase progression checkpoint requires rad3, rad26, and cds1. (A) rad+ cells (_h_− cyr1 sxa2) and rad3 mutant cells (h_−_cyr1 sxa2 rad3) were synchronized in G1 by centrifugal elutriation (collecting early G2 cells) followed by exposure to P factor. Cells were released from P factor-induced G1 arrest in either the presence or absence of 0.033% MMS and S-phase progression followed by FACS. Attempts to synchronize other checkpoint mutants this way were complicated by the morphological changes associated with the cyr1 sxa2 background. (B) Wild-type, rad3, cds1, chk1, rad26, and rad26-T12 cells were synchronized in G1 by nitrogen starvation, released into the cell cycle in the presence or absence of 0.033% MMS and followed by FACS. As before, rad3 cells are unable to slow S phase in response to DNA damage. Similarly, cds1 and rad26 are required for this checkpoint, whereas chk1 is not. Cells carrying the rad26.T12 mutation also have a defect in this checkpoint.
Figure 7
Mitotic arrest in cds1 mutants is dependent on Chk1 activation. (A) Synchronous cultures of appropriate strains were tested for the mitotic checkpoint in response to HU as in Fig. 1. (•) −Hu; (□) + HU. (B) The phosphorylation-dependent mobility shift of Chk1 kinase was assayed in cds1+ and cds1 null mutant cells containing an integrated triple HA-tagged chk1 gene (Walworth and Bernards 1996). Samples from asynchronous cultures treated with 12 m
m
HU were taken at the times indicated. Chk1 was detected, as described previously (Walworth and Bernards 1996), with anti-HA monoclonal. In cds1+ cells, Chk1 remains unphosphorylated, whereas in cds1 null mutant cells Chk1 is rapidly phosphorylated.
Figure 8
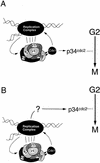
Model for the function of Cds1 in the S-phase arrest response. (A) Checkpoint Rad-dependent activation of Cds1 by perturbations to S-phase acts to ensure both S-phase arrest, by phosphorylating components of the replication complex, and mitotic arrest by phosphorylating a substrate that leads to inactivation of the mitotic apparatus. (B) Cds1 only acts to phosphorylate and stabilize the replication machinery. This prevents inappropriate DNA damage and maintains the structure(s) that are activating a Cds1 independent mitotic arrest signal. This signal would also be independent of the checkpoint Rad proteins, until such time as replication was perturbed, when maintenance of the appropriate replication structures becomes a prerequisite.
Similar articles
- Analysis of Rad3 and Chk1 protein kinases defines different checkpoint responses.
Martinho RG, Lindsay HD, Flaggs G, DeMaggio AJ, Hoekstra MF, Carr AM, Bentley NJ. Martinho RG, et al. EMBO J. 1998 Dec 15;17(24):7239-49. doi: 10.1093/emboj/17.24.7239. EMBO J. 1998. PMID: 9857181 Free PMC article. - A novel mutant allele of Schizosaccharomyces pombe rad26 defective in monitoring S-phase progression to prevent premature mitosis.
Uchiyama M, Galli I, Griffiths DJ, Wang TS. Uchiyama M, et al. Mol Cell Biol. 1997 Jun;17(6):3103-15. doi: 10.1128/MCB.17.6.3103. Mol Cell Biol. 1997. PMID: 9154809 Free PMC article. - Basis for the checkpoint signal specificity that regulates Chk1 and Cds1 protein kinases.
Brondello JM, Boddy MN, Furnari B, Russell P. Brondello JM, et al. Mol Cell Biol. 1999 Jun;19(6):4262-9. doi: 10.1128/MCB.19.6.4262. Mol Cell Biol. 1999. PMID: 10330167 Free PMC article. - Checking in on Cds1 (Chk2): A checkpoint kinase and tumor suppressor.
McGowan CH. McGowan CH. Bioessays. 2002 Jun;24(6):502-11. doi: 10.1002/bies.10101. Bioessays. 2002. PMID: 12111733 Review. - Cdc7 kinases (DDKs) and checkpoint responses: lessons from two yeasts.
Duncker BP, Brown GW. Duncker BP, et al. Mutat Res. 2003 Nov 27;532(1-2):21-7. doi: 10.1016/j.mrfmmm.2003.08.007. Mutat Res. 2003. PMID: 14643426 Review.
Cited by
- Meiotic DNA replication checkpoint control in fission yeast.
Murakami H, Nurse P. Murakami H, et al. Genes Dev. 1999 Oct 1;13(19):2581-93. doi: 10.1101/gad.13.19.2581. Genes Dev. 1999. PMID: 10521402 Free PMC article. - Postreplication gaps at UV lesions are signals for checkpoint activation.
Callegari AJ, Clark E, Pneuman A, Kelly TJ. Callegari AJ, et al. Proc Natl Acad Sci U S A. 2010 May 4;107(18):8219-24. doi: 10.1073/pnas.1003449107. Epub 2010 Apr 19. Proc Natl Acad Sci U S A. 2010. PMID: 20404181 Free PMC article. - ATRIP oligomerization is required for ATR-dependent checkpoint signaling.
Ball HL, Cortez D. Ball HL, et al. J Biol Chem. 2005 Sep 9;280(36):31390-6. doi: 10.1074/jbc.M504961200. Epub 2005 Jul 15. J Biol Chem. 2005. PMID: 16027118 Free PMC article. - Response of Xenopus Cds1 in cell-free extracts to DNA templates with double-stranded ends.
Guo Z, Dunphy WG. Guo Z, et al. Mol Biol Cell. 2000 May;11(5):1535-46. doi: 10.1091/mbc.11.5.1535. Mol Biol Cell. 2000. PMID: 10793133 Free PMC article. - Chk1-deficient tumour cells are viable but exhibit multiple checkpoint and survival defects.
Zachos G, Rainey MD, Gillespie DA. Zachos G, et al. EMBO J. 2003 Feb 3;22(3):713-23. doi: 10.1093/emboj/cdg060. EMBO J. 2003. PMID: 12554671 Free PMC article.
References
- Allen JB, Zhou Z, Siede W, Friedberg EC, Elledge SJ. The SAD1/RAD53 protein kinase controls multiple checkpoints and DNA damage-induced transcription in yeast. Genes & Dev. 1994;8:2401–2415. - PubMed
- Barbet NC, Carr AM. Fission yeast wee1 protein kinase is not required for DNA damage-dependent mitotic arrest. Nature. 1993;364:824–827. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases