Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture - PubMed (original) (raw)
Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture
A Rao et al. J Neurosci. 1998.
Abstract
To determine their roles in the assembly of glutamatergic postsynaptic sites, we studied the distributions of NMDA- and AMPA-type glutamate receptors; the NMDA receptor-interacting proteins alpha-actinin-2, PSD-95, and chapsyn; and the PSD-95-associated protein GKAP during the development of hippocampal neurons in culture. NMDA receptors first formed nonsynaptic proximal dendrite shaft clusters within 2-5 d. AMPA receptors were diffuse at this stage and began to cluster on spines at 9-10 d. NMDA receptor clusters remained partially nonsynaptic and mainly distinct from AMPA receptor clusters until after 3 weeks in culture, when the two began to colocalize at spiny synaptic sites. Thus, the localization of NMDA and AMPA receptors must be regulated by different mechanisms. alpha-Actinin-2 colocalized with the NMDA receptor only at spiny synaptic clusters, but not at shaft nonsynaptic or synaptic clusters, suggesting a modulatory role in the anchoring of NMDA receptor at spines. PSD-95, chapsyn, and GKAP were present at some, but not all, nonsynaptic NMDA receptor clusters during the first 2 weeks, indicating that none is essential for NMDA receptor cluster formation. When NMDA receptor clusters became synaptic, PSD-95 and GKAP were always present, consistent with an essential function in synaptic localization of NMDA receptors. Furthermore, PSD-95 and GKAP clustered opposite presynaptic terminals several days before either NMDA or AMPA receptors clustered at these presumptive postsynaptic sites. These results suggest that synapse development proceeds by formation of a postsynaptic scaffold containing PSD-95 and GKAP in concert with presynaptic vesicle clustering, followed by regulated attachment of glutamate receptor subtypes to this scaffold.
Figures
Fig. 1.
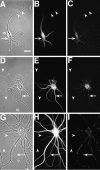
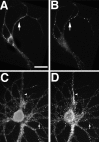
NR1 puncta are restricted to the somatodendritic domain in hippocampal neurons in culture. Hippocampal cultures were fixed at 3 (A_–_C), 9 (D_–_F), and 23 (G_–_I) d and immunostained for the dendritic marker MAP2 (B, E,H) and NR1 (C, F,I). In the phase-contrast images (A, D, G), axons can be seen traversing the substrate (arrowheads), whereas in the paired immunolabeled images no immunostaining can be observed in these processes. Clusters of NR1 (arrows) are evident in the soma and proximal dendrites of the isolated 3-d-old cell in the absence of axonal contacts. At later stages, NR1 clusters move further into the dendrite shafts (F) and by 3 weeks (I) are at the tips of dendrites. Scale bar, 20 μm.
Fig. 2.
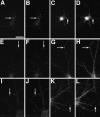
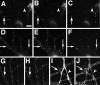
NMDA- and AMPA-type receptors cluster separately until late in development. Cultures fixed at 5 (A_–_C), 14 (D_–_F), and 21 (G_–_L) d in vitro were immunostained for NR1 (A, D,G, J), GluR1 (B,E, H, K), and the presynaptic marker synaptophysin (C, F,I, L). In the 5 d cell, NR1 (A) forms clusters at sites distinct from the synaptic sites indicated by synaptophysin clusters (C). GluR1 staining is detectable but diffuse (B). At 14 d, NR1 clusters have moved out into the proximal dendrite (D) and are still distinct from presynaptic sites (F). GluR1 forms aggregates at spiny sites along the full length of the dendrites (E), always apposed to presynaptic sites (F). By 21 d, two additional patterns of NR1 staining are apparent. Figure legend continues. NR1 can form arrays of large, brightly labeled clusters at the distal dendrite shaft (G) or can cluster at spiny sites throughout the dendritic arbor (J). The distal dendrite shaft NR1 clusters are mostly nonsynaptic but sometimes synaptic (compare_G_ and I) and lack concentrations of GluR1, which is still clustered in spines (H). The spiny NR1 clusters (J) are apposed to presynaptic sites (L) and often colocalize with GluR1 clusters (K). Scale bar, 10 μm.Insets show magnified regions from the full panels.
Fig. 3.
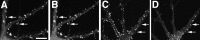
NR2A and NR2B are present at all NR1 clusters, including synaptic and nonsynaptic types. Hippocampal cultures fixed at 5 (A_–_D), 12 (E_–_H), 21 (I,J), and 28 d (K,L) were immunostained for NR1 (A,C, E, G, I,K) and NR2A (B, F,J) or NR2B (D, H,L). The two NR2 receptor subunits colocalized with NR1 at all of these stages of development, corresponding to the proximal shaft, distal shaft, and spiny clustering patterns. _Arrows_indicate prominent NR1 clusters, which show co-localized NR2 subunits. Scale bar, 10 μm.
Fig. 4.
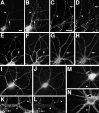
PSD-95 is present at excitatory synaptic sites, but not at inhibitory synapses. Hippocampal neurons fixed at 21 d in culture were immunostained for PSD-95 (A,C) and either GluR1 (B) or the GABAA receptor β2/3 subunit (D). PSD-95 clusters colocalized with GluR1-labeled excitatory synapses (indicated by arrows in A,B), but not with the GABAA receptor-labeled inhibitory synapses (arrows in C,D). Scale bar, 10 μm.
Fig. 5.
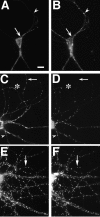
PSD-95 and NR1 can cluster separately early in development but colocalize in older neurons. Neurons fixed at 3 (A, B), 14 (C,D), and 35 (E, F) d in culture were immunostained for NR1 (A,C, E) and PSD-95 (B,D, F). Nonsynaptic-type clusters of NR1 in the 3 and 14 d cells can form in the absence of PSD-95 staining (A–D, arrows), indicating that PSD95 is not necessary for clustering at nonsynaptic sites. Frequently, as in other sites on these cells, NR1 and PSD-95 do colocalize at these clusters. Clustering of PSD-95 without NR1 (A,B, arrowheads) indicates that PSD-95 clustering is not sufficient to induce NR1 clustering at the same site. The asterisk in C and D_shows distal dendrite NR1 clusters that do colocalize with PSD-95 and may be synaptic. In more mature neurons, at a stage when NR1 is spiny and probably synaptic (compare with Fig. 2_J,L), NR1 and PSD-95 clusters are colocalized almost completely (E, F, arrows). Scale bar, 10 μm.
Fig. 6.
Chapsyn-110 can cocluster with NR1 but also is concentrated at the axon initial segment. Neurons fixed at 5 (A, B) and 35 (C,D) d in culture were immunostained for NR1 (A, C) and chapsyn (B,D). Like PSD-95, chapsyn clustered with NR1 at some sites early in development (A, B,arrow) but also could cluster separately. In mature neurons, chapsyn coclustered with spiny NR1 (C,D, arrow) but also was concentrated at the axon initial segment without concentrations of NR1 (C, D, arrowhead). Scale bar, 20 μm.
Fig. 7.
GKAP and PSD-95 are the earliest components of developing glutamatergic postsynaptic sites. Neurons fixed at 5 (A_–_C), 10 (D_–_F), and 19 (G_–_J) d in culture were immunostained for GKAP (A, N-terminal antibody;D, G, I, C-terminal antibody), PSD-95 (B, E,H, J), and the presynaptic marker SV2 (C, F). GKAP and PSD-95 were always colocalized (arrows in A and_B_, D and E,G and H). At very early stages GKAP and PSD-95 clusters were prominent, apposed to some presynaptic sites (A_–_C, arrow) but faint at others (A_–_C,arrowhead). By 10 d, GKAP and PSD-95 appeared at almost all synaptic sites (D_–_F,arrows) as well as some nonsynaptic sites. At these stages NR1 is completely nonsynaptic, and GluR1 is clustered in very few cells (see Results and Fig. 2), so GKAP and PSD-95 must form clusters at synaptic sites before glutamate receptors. In mature neurons GKAP still coclusters with PSD-95 at all sites but is enriched in GABAergic interneurons, when compared with pyramidal neurons (G_–_J). GKAP and PSD-95 colocalize on the spines of mature pyramidal neurons (G,H, arrow). In I and_J_, the dendrites of a GABAergic cell cross the field vertically, whereas the dendrites of a pyramidal cell cross horizontally. GKAP staining in the GABAergic cell dendrite (I, arrowhead) is far brighter than in the pyramidal cell (I, arrow), when compared with the uniform levels of PSD95 staining at the same sites (J). Scale bar, 10 μm.
Fig. 8.
α-Actinin-2 is associated with dendritic spines. NR1 (A, C) and α-actinin-2 (B, D) were not colocalized at shaft clusters of NR1 (arrows) in either 19 (A,B) or 28 (C, D) d cultured hippocampal neurons. α-Actinin-2 formed spiny clusters all over the dendritic tree at 19 d (B) and earlier (F). NR1 was not present at most of these spiny clusters (A_–_D,arrowheads), but when NR1 clusters became spiny, they colocalized with α-actinin-2 (C, D,asterisk). GluR1 (E, G) clusters colocalized with α-actinin-2 (F,H) at dendritic spines from the earliest time GluR1 clusters were visible (10 d cell in E,F) and thereafter (21 d cell in G,H). In GABAergic cells in the culture (I_–_L), GluR1 clustered on dendrite shafts (I, K). α-Actinin-2 (J, L) was not present at these shaft GluR1 clusters. In K and L, GABAergic cell dendrites cross from the right(arrowheads) to intersect with pyramidal neuron dendrites coming from the left (arrow). α-Actinin-2 is present at the spiny GluR1 clusters of the pyramidal cell (arrow), but not at the shaft GluR1 clusters of the GABA cell (arrowhead). Frequently, α-actinin-2 also formed elongated clusters in the soma and dendrite core (M, arrow), which were not at synaptic sites (as indicated by synaptophysin staining in_N_). Arrowheads indicate synaptic sites. Scale bars: in A, 10 μm (for all panels except_C_, D, K,L); in C, 10 μm for C, D, K, L).
Fig. 9.
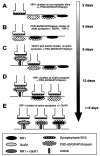
Summary of the stages of development of excitatory postsynaptic sites on hippocampal pyramidal neurons, as indicated by the molecular markers that were used in this study. The time line indicates the earliest time in culture at which each event was observed; many of these events occurred over a span of days to weeks.A, NMDA receptors were clustered from the earliest stages in development at nonsynaptic sites in the soma and proximal dendrite shaft; some clusters colocalized with PSD-95, GKAP, and chapsyn, and some did not. B, PSD-95, GKAP, and chapsyn formed clusters within the first week in culture at dendrite shaft synapses lacking clusters of either NMDA or AMPA receptors.C, α-Actinin-2 formed synaptic clusters in the second week in culture but only at spine synapses colocalizing with AMPA receptor, but not NMDA receptor clusters. D, During the second and third week in culture, NMDA receptor clusters were predominantly at fine terminal branches of the dendritic tree, many of them colocalizing with PSD-95, GKAP, and chapsyn; some of these were synaptic. E, Finally, only beginning at 3 weeks did NMDA receptor clusters become localized primarily at dendritic spines throughout the extent of the dendritic arborization, where they often colocalized with AMPA receptor clusters and always with α-actinin-2, PSD-95, GKAP, and chapsyn.
Similar articles
- Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors.
Allison DW, Gelfand VI, Spector I, Craig AM. Allison DW, et al. J Neurosci. 1998 Apr 1;18(7):2423-36. doi: 10.1523/JNEUROSCI.18-07-02423.1998. J Neurosci. 1998. PMID: 9502803 Free PMC article. - Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons.
Rao A, Cha EM, Craig AM. Rao A, et al. J Neurosci. 2000 Nov 15;20(22):8344-53. doi: 10.1523/JNEUROSCI.20-22-08344.2000. J Neurosci. 2000. PMID: 11069941 Free PMC article. - A functional role of postsynaptic density-95-guanylate kinase-associated protein complex in regulating Shank assembly and stability to synapses.
Romorini S, Piccoli G, Jiang M, Grossano P, Tonna N, Passafaro M, Zhang M, Sala C. Romorini S, et al. J Neurosci. 2004 Oct 20;24(42):9391-404. doi: 10.1523/JNEUROSCI.3314-04.2004. J Neurosci. 2004. PMID: 15496675 Free PMC article. - PSD-95-like membrane associated guanylate kinases (PSD-MAGUKs) and synaptic plasticity.
Xu W. Xu W. Curr Opin Neurobiol. 2011 Apr;21(2):306-12. doi: 10.1016/j.conb.2011.03.001. Epub 2011 Mar 28. Curr Opin Neurobiol. 2011. PMID: 21450454 Free PMC article. Review. - Roles of postsynaptic density-95/synapse-associated protein 90 and its interacting proteins in the organization of synapses.
Hata Y, Takai Y. Hata Y, et al. Cell Mol Life Sci. 1999 Oct 30;56(5-6):461-72. doi: 10.1007/s000180050445. Cell Mol Life Sci. 1999. PMID: 11212298 Free PMC article. Review.
Cited by
- Pin1 Modulates the Synaptic Content of NMDA Receptors via Prolyl-Isomerization of PSD-95.
Antonelli R, De Filippo R, Middei S, Stancheva S, Pastore B, Ammassari-Teule M, Barberis A, Cherubini E, Zacchi P. Antonelli R, et al. J Neurosci. 2016 May 18;36(20):5437-47. doi: 10.1523/JNEUROSCI.3124-15.2016. J Neurosci. 2016. PMID: 27194325 Free PMC article. - The cytoskeletal protein alpha-actinin regulates acid-sensing ion channel 1a through a C-terminal interaction.
Schnizler MK, Schnizler K, Zha XM, Hall DD, Wemmie JA, Hell JW, Welsh MJ. Schnizler MK, et al. J Biol Chem. 2009 Jan 30;284(5):2697-2705. doi: 10.1074/jbc.M805110200. Epub 2008 Nov 21. J Biol Chem. 2009. PMID: 19028690 Free PMC article. - Silent synapses in developing rat nucleus tractus solitarii have AMPA receptors.
Balland B, Lachamp P, Kessler JP, Tell F. Balland B, et al. J Neurosci. 2008 Apr 30;28(18):4624-34. doi: 10.1523/JNEUROSCI.5355-07.2008. J Neurosci. 2008. PMID: 18448639 Free PMC article. - Citron binds to PSD-95 at glutamatergic synapses on inhibitory neurons in the hippocampus.
Zhang W, Vazquez L, Apperson M, Kennedy MB. Zhang W, et al. J Neurosci. 1999 Jan 1;19(1):96-108. doi: 10.1523/JNEUROSCI.19-01-00096.1999. J Neurosci. 1999. PMID: 9870942 Free PMC article. - Simultaneous analysis of dendritic spine density, morphology and excitatory glutamate receptors during neuron maturation in vitro by quantitative immunocytochemistry.
Nwabuisi-Heath E, LaDu MJ, Yu C. Nwabuisi-Heath E, et al. J Neurosci Methods. 2012 Jun 15;207(2):137-47. doi: 10.1016/j.jneumeth.2012.04.003. Epub 2012 Apr 10. J Neurosci Methods. 2012. PMID: 22521963 Free PMC article.
References
- Apel ED, Glass DJ, Moscoso LM, Yancopoulos GD, Sanes JR. Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron. 1997;18:623–635. - PubMed
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–425. - PubMed
- Bekkers JM, Stevens CF. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989;341:230–233. - PubMed
- Benson DL, Watkins FH, Steward O, Banker G. Characterization of GABAergic neurons in hippocampal cell cultures. J Neurocytol. 1994;23:279–295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources