Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent - PubMed (original) (raw)
Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent
B M Mullock et al. J Cell Biol. 1998.
Abstract
Using a cell-free content mixing assay containing rat liver endosomes and lysosomes in the presence of pig brain cytosol, we demonstrated that after incubation at 37 degrees C, late endosome-lysosome hybrid organelles were formed, which could be isolated by density gradient centrifugation. ImmunoEM showed that the hybrids contained both an endocytosed marker and a lysosomal enzyme. Formation of the hybrid organelles appeared not to require vesicular transport between late endosomes and lysosomes but occurred as a result of direct fusion. Hybrid organelles with similar properties were isolated directly from rat liver homogenates and thus were not an artifact of cell-free incubations. Direct fusion between late endosomes and lysosomes was an N-ethylmaleimide-sensitive factor-dependent event and was inhibited by GDP-dissociation inhibitor, indicating a requirement for a rab protein. We suggest that in cells, delivery of endocytosed ligands to an organelle where proteolytic digestion occurs is mediated by direct fusion of late endosomes with lysosomes. The consequences of this fusion to the maintenance and function of lysosomes are discussed.
Figures
Figure 1
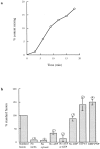
Characterization of content mixing between late endosomes and lysosomes. (a) Time course under standard incubation conditions, i.e., in the presence of cytosol, an ATP-regenerating system, 1 mM ATP, and 1 mM GTP. Results are expressed at each time point as the percentage of the total immunoprecipitable counts that had been formed within membranous compartments (i.e., which were immunoprecipitable in the presence of biocytin). (b) Requirements for the content mixing reaction. Results are expressed as percentages of the content mixing obtained on the same day under the standard conditions, i.e., in the presence of cytosol, an ATP-regenerating system, 1 mM ATP, and 1 mM GTP. 0.2 mM GMP-PNP or guanosine 5′-_O_-(3-thiotriphosphate (GTPγS) replaced GTP where indicated. incbn, incubation.
Figure 2
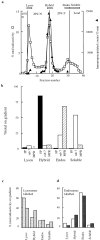
Separation of late endosome–lysosome hybrids on a step gradient. (a) Distribution of total and immunoprecipitable radiolabels after a standard content mixing assay containing 125I-bpIgA–loaded lysosomes and Av-ASF–loaded late endosomes incubated for 10 min at 37°C; □, percentage of total radioactivity on gradient; ▴, radioactivity in the peak fractions, immunoprecipitable with antiavidin. (Radioactive counts were routinely collected for 15 min and corrected for background.) The radioactivity at the light end of the step gradient peaking around fraction 30 was not precipitable with trichloroacetic acid, indicating that it represented soluble degradation products. (b) Distribution of immunoprecipitable radioactivity (FP, fusion product), immunoblotted rab 7, and immunoblotted MPR on the step gradient run under the same conditions as in a. The four regions of the gradient are shown at the top of a. *, no detectable immunoblotted MPR in the Lysos region. (c) Separate experiment showing distribution of total radioactivity before ( ) and after (
) and after ( ) the incubation of the content mixing assay (lysosomes loaded with 125I-bpIgA). (d) Distribution of total radioactivity before (□) and after (▪) incubation of 125I-Av-ASF–loaded late endosomes with unlabeled lysosomes under the conditions of the content mixing assay. Lysos, lysosomes; Endos, endosomes; N, Nycodenz; F, Ficoll.
) the incubation of the content mixing assay (lysosomes loaded with 125I-bpIgA). (d) Distribution of total radioactivity before (□) and after (▪) incubation of 125I-Av-ASF–loaded late endosomes with unlabeled lysosomes under the conditions of the content mixing assay. Lysos, lysosomes; Endos, endosomes; N, Nycodenz; F, Ficoll.
Figure 3
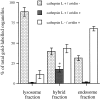
Quantitation of cathepsin L and avidin immunolabeled organelles of ultrathin frozen sections from the fractions illustrated in Fig. 4. Results are presented as a mean ± the range (lysosome fraction) or SEM (hybrid and endosome fractions). Cathepsin L+/avidin+ structures were significantly enriched in the hybrid fraction compared to the lysosome and endosome fractions, * P < 0.01. The cathepsin L+/avidin− structures in the lysosome fraction were electron dense, whereas similarly labeled structures in the endosome fraction were electron lucent, suggesting that the latter may be vesicles and organelles from the route of delivery of newly synthesized cathepsin to lysosomes.
Figure 4
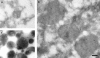
Immunoelectron microscopy identifying avidin (8-nm gold) and cathepsin L (15-nm gold) immunoreactivity in organelles from late endosome, lysosome, and hybrid fractions. (a) A late endosome peak fraction from the preparative 1–22% Ficoll gradient showing an endosomal structure containing Av-ASF. (b) Hybrid organelles formed after the content mixing assay incubation accumulated at the 20% Nycodenz/20% Ficoll interface of the step gradient (see Fig. 2). These membrane-bounded organelles from the interface peak fractions were immunolabeled with antibodies to both cathepsin L and avidin. (c) Electron-dense lysosomes that could be immunolabeled with anti–cathepsin L antibodies accumulated at the 45%/20% Nycodenz interface of the step gradient after the content mixing assay. Bar, 200 nm.
Figure 5
Content mixing after preincubation of late endosomes for 5 min. Late endosomes were preincubated (preinc) at 37°C for 5 min in cytosol with an ATP-regenerating system (E) and 1 mM GTP, with omissions as shown or with 200 μM GMP-PNP replacing GTP. As a control in STM, late endosomes were preincubated at 37°C for 5 min in STM alone.
Figure 6
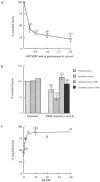
Content mixing requires NSF. (a) Dose-dependent inhibition of content mixing by NEM treatment of cytosol. Cytosol was pretreated with NEM at 4°C for 15 min before quenching with DTT. (b) Destruction of NSF and replacement with recombinant NSF. Cytosol was incubated at 37°C for 15 min in the absence of ATP to destroy NSF; late endosomes (LE) and lysosomes (lys) were separately treated with 2 mM NEM at 4°C for 15 min before quenching with DTT. Recombinant NSF was added to 10 nM. (c) Dose dependence of recombinant NSF addition. Cytosol was NSF-depleted by incubation and late endosomes and lysosomes were NEM treated as in b.
Figure 7
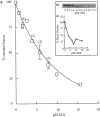
Inhibition of content mixing by GDI. (a) Content mixing in the presence of GDI. (b) Removal of rab 7 from the membranes in a representative content mixing assay by GDI. (c) Quantitation of the data shown in b.
Figure 8
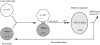
Diagrammatic representation of the direct fusion of late endosomes with lysosomes to form a hybrid organelle of intermediate density. Binding of late endosomes to lysosomes may be mediated by filamentous structures that have been observed by electron microscopy. The formation of the hybrid organelle requires NSF and SNAPs and is inhibited by GDI, although the exact step at which they function and their order of interaction are unknown. Previous experiments with NRK cells (Bright et al., 1997) suggest that dense-core lysosomes may reform from hybrid organelles. This requires recondensation of contents and probably retrieval of membrane to an unknown location, possibly to an earlier site in the endocytic pathway. cat L, cathepsin L-positive structures.
Similar articles
- The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles.
Pryor PR, Mullock BM, Bright NA, Gray SR, Luzio JP. Pryor PR, et al. J Cell Biol. 2000 May 29;149(5):1053-62. doi: 10.1083/jcb.149.5.1053. J Cell Biol. 2000. PMID: 10831609 Free PMC article. - Live Salmonella recruits N-ethylmaleimide-sensitive fusion protein on phagosomal membrane and promotes fusion with early endosome.
Mukherjee K, Siddiqi SA, Hashim S, Raje M, Basu SK, Mukhopadhyay A. Mukherjee K, et al. J Cell Biol. 2000 Feb 21;148(4):741-53. doi: 10.1083/jcb.148.4.741. J Cell Biol. 2000. PMID: 10684255 Free PMC article. - Relationship between endosomes and lysosomes.
Luzio JP, Mullock BM, Pryor PR, Lindsay MR, James DE, Piper RC. Luzio JP, et al. Biochem Soc Trans. 2001 Aug;29(Pt 4):476-80. doi: 10.1042/bst0290476. Biochem Soc Trans. 2001. PMID: 11498012 Review. - Reconstitution of phagosome-lysosome fusion in streptolysin O-permeabilized cells.
Funato K, Beron W, Yang CZ, Mukhopadhyay A, Stahl PD. Funato K, et al. J Biol Chem. 1997 Jun 27;272(26):16147-51. doi: 10.1074/jbc.272.26.16147. J Biol Chem. 1997. PMID: 9195911 - Regulation of endosome fusion.
Mills IG, Jones AT, Clague MJ. Mills IG, et al. Mol Membr Biol. 1999 Jan-Mar;16(1):73-9. doi: 10.1080/096876899294788. Mol Membr Biol. 1999. PMID: 10332740 Review.
Cited by
- Vps34 regulates Rab7 and late endocytic trafficking through recruitment of the GTPase-activating protein Armus.
Jaber N, Mohd-Naim N, Wang Z, DeLeon JL, Kim S, Zhong H, Sheshadri N, Dou Z, Edinger AL, Du G, Braga VM, Zong WX. Jaber N, et al. J Cell Sci. 2016 Dec 1;129(23):4424-4435. doi: 10.1242/jcs.192260. Epub 2016 Oct 28. J Cell Sci. 2016. PMID: 27793976 Free PMC article. - Loss of HD-PTP function results in lipodystrophy, defective cellular signaling and altered lipid homeostasis.
Schultz DF, Davies BA, Payne JA, Martin CP, Minard AY, Childs BG, Zhang C, Jeganathan KB, Sturmlechner I, White TA, de Bruin A, Harkema L, Chen H, Davies MA, Jachim S, LeBrasseur NK, Piper RC, Li H, Baker DJ, van Deursen J, Billadeau DD, Katzmann DJ. Schultz DF, et al. J Cell Sci. 2024 Sep 15;137(18):jcs262032. doi: 10.1242/jcs.262032. Epub 2024 Sep 27. J Cell Sci. 2024. PMID: 39155850 - Loss of AP-5 results in accumulation of aberrant endolysosomes: defining a new type of lysosomal storage disease.
Hirst J, Edgar JR, Esteves T, Darios F, Madeo M, Chang J, Roda RH, Dürr A, Anheim M, Gellera C, Li J, Züchner S, Mariotti C, Stevanin G, Blackstone C, Kruer MC, Robinson MS. Hirst J, et al. Hum Mol Genet. 2015 Sep 1;24(17):4984-96. doi: 10.1093/hmg/ddv220. Epub 2015 Jun 17. Hum Mol Genet. 2015. PMID: 26085577 Free PMC article. - HOPS-dependent lysosomal fusion controls Rab19 availability for ciliogenesis in polarized epithelial cells.
Hoffman HK, Prekeris R. Hoffman HK, et al. bioRxiv [Preprint]. 2023 Feb 8:2023.02.07.527563. doi: 10.1101/2023.02.07.527563. bioRxiv. 2023. PMID: 36798155 Free PMC article. Updated. Preprint. - Detection and quantification of the vacuolar H+ATPase using the Legionella effector protein SidK.
Maxson ME, Abbas YM, Wu JZ, Plumb JD, Grinstein S, Rubinstein JL. Maxson ME, et al. J Cell Biol. 2022 Mar 7;221(3):e202107174. doi: 10.1083/jcb.202107174. Epub 2022 Jan 13. J Cell Biol. 2022. PMID: 35024770 Free PMC article.
References
- Acharya U, Jacobs R, Peters J, Watson N, Farquhar MG, Malhotra V. The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell. 1995;82:895–904. - PubMed
- Blommaart EF, Krause U, Schellens JPM, Vreeling-Sindelarova H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem. 1997;243:240–246. - PubMed