LFA-1-mediated adhesion is regulated by cytoskeletal restraint and by a Ca2+-dependent protease, calpain - PubMed (original) (raw)
LFA-1-mediated adhesion is regulated by cytoskeletal restraint and by a Ca2+-dependent protease, calpain
M P Stewart et al. J Cell Biol. 1998.
Abstract
The activity of integrins on leukocytes is kept under tight control to avoid inappropriate adhesion while these cells are circulating in blood or migrating through tissues. Using lymphocyte function-associated antigen-1 (LFA-1) on T cells as a model, we have investigated adhesion to ligand intercellular adhesion molecule-1 induced by the Ca2+ mobilizers, ionomycin, 2, 5-di-t-butylhydroquinone, and thapsigargin, and the well studied stimulators such as phorbol ester and cross-linking of the antigen-specific T cell receptor (TCR)-CD3 complex. We report here that after exposure of T cells to these agonists, integrin is released from cytoskeletal control by the Ca2+-induced activation of a calpain-like enzyme, and adhesive contact between cells is strengthened by means of the clustering of mobilized LFA-1 on the membrane. We propose that methods of leukocyte stimulation that cause Ca2+ fluxes induce LFA-1 adhesion by regulation of calpain activity. These findings suggest a mechanism whereby engagement of the TCR could promote adhesion strengthening at an early stage of interaction with an antigen-presenting cell.
Figures
Figure 1
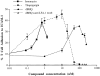
Ca2+ mobilizers induce adhesion of T cell LFA-1 to ICAM-1Fc. T cells were treated with ionomycin, thapsigargin, and dBHQ at the indicated concentrations and incubated on ICAM-1Fc–coated 96-well plates for 30 min at 37°C. The proportion of adhering cells was calculated as a percentage of total cells added per well. For all stimulants, adhesion was reduced to background levels by means of anti–LFA-1 mAb used at 10 μg/ml. An experiment representative of five similar experiments is presented.
Figure 2
Thapsigargin and ionomycin-induced adhesion is dependent on extracellular Ca2+. T cells were preincubated with 100 μM SK&F 96365 in RPMI for 30 min at 37°C, and then incubated with thapsigargin (5 μM) and ionomycin (0.7 μM) on ICAM-1Fc–coated 96-well plates for a further 30 min at 37°C. The proportion of adhering cells was calculated as a percentage of total cells added per well. For all stimulants, adhesion was reduced to background levels by anti–LFA-1 mAb used at 10 μg/ ml. An experiment representative of three is presented.
Figure 3
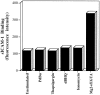
Thapsigargin, ionomcyin, and dBHQ do not induce binding of soluble ICAM-1 to LFA-1. Soluble ICAM-1Fc at 1 mg/ml (or 4.5 μM) was incubated with thapsigargin (5 μM), ionomcyin (0.7 μM), dBHQ (50 μM), PdBu (50 nM), and Mg2+/ EGTA (5 mM/1 mM)–stimulated cells for 30 min at 37°C (Stewart et al., 1996). Bound sICAM-1 was detected with FITC-conjugated goat anti–human (Fc specific) Ab and analyzed by flow cytometry. Results are expressed as median fluorescence intensity. An experiment representative of three is presented.
Figure 4
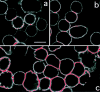
Thapsigargin stimulates LFA-1 clustering. T cells adhered onto poly-L-lysine were (a) unstimulated; (b) treated with 5 mM Mg2+/1 mM EGTA; or (c) treated with 5 μM thapsigargin for 30 min at 37°C. LFA-1 was detected with FITC-conjugated LFA-1–specific mAb and analyzed by confocal microscopy. Red color indicates fluorescence intensity exceeding a preset pixel value (see Materials and Methods). This setting remained constant between samples. Bar, 10 μm.
Figure 7
LFA-1 clustering induced by thapsigargin and anti-TCR–CD3 triggering is inhibited by calpeptin. Clustering of LFA-1 induced by 5 μM thapsigargin (a) and 10 μg/ml anti-CD3 mAb (b) was prevented by preincubation with 100 μg/ml calpeptin, (c and d, respectively), for 30 min at 37°C. Bar, 10 μm.
Figure 5
Thapsigargin stimulates LFA-1 clustering through a cytoskeletal release mechanism. T cells adhered onto poly-L-lysine were either (a) treated with 5 μM thapsigargin for 30 min at 37°C, or (b) preincubated with 1 μM jasplakinolide for 30 min at 37°C, before treatment as in a. LFA-1 was detected with FITC-conjugated LFA-1 specific mAb and analyzed by confocal microscopy as in Fig. 4. Bar, 10 μm.
Figure 6
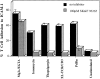
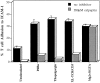
Calpain inhibitors block LFA-1–mediated adhesion induced by agonists acting intracellularly. Cells were preincubated with or without 280 μM (100 μg/ml) calpeptin for 30 min at 37°C before analysis in a T cell adhesion assay where stimulants were 50 nM PdBu, 5 μM thapsigargin, 10 μg/ml anti-CD3 mAb G19.4, 5 mM Mg2+/1 mM EGTA.
Similar articles
- LFA-1 integrin and the microtubular cytoskeleton are involved in the Ca(2)(+)-mediated regulation of the activity of the tyrosine kinase PYK2 in T cells.
Rodríguez-Fernández JL, Sánchez-Martín L, de Frutos CA, Sancho D, Robinson M, Sánchez-Madrid F, Cabañas C. Rodríguez-Fernández JL, et al. J Leukoc Biol. 2002 Mar;71(3):520-30. J Leukoc Biol. 2002. PMID: 11867690 - Calpain 2 controls turnover of LFA-1 adhesions on migrating T lymphocytes.
Svensson L, McDowall A, Giles KM, Stanley P, Feske S, Hogg N. Svensson L, et al. PLoS One. 2010 Nov 30;5(11):e15090. doi: 10.1371/journal.pone.0015090. PLoS One. 2010. PMID: 21152086 Free PMC article. - Activation of leukocyte function-associated antigen-1 on adult T-cell leukemia cells.
Tanaka Y. Tanaka Y. Leuk Lymphoma. 1999 Dec;36(1-2):15-23. doi: 10.3109/10428199909145945. Leuk Lymphoma. 1999. PMID: 10613446 Review. - The CD2-LFA-3 and LFA-1-ICAM pathways: relevance to T-cell recognition.
Makgoba MW, Sanders ME, Shaw S. Makgoba MW, et al. Immunol Today. 1989 Dec;10(12):417-22. doi: 10.1016/0167-5699(89)90039-X. Immunol Today. 1989. PMID: 2482743 Review.
Cited by
- Foxp3-mediated blockage of ryanodine receptor 2 underlies contact-based suppression by regulatory T cells.
Wang X, Geng S, Meng J, Kang N, Liu X, Xu Y, Lyu H, Xu Y, Xu X, Song X, Zhang B, Wang X, Nuerbulati N, Zhang Z, Zhai D, Mao X, Sun R, Wang X, Wang R, Guo J, Chen SRW, Zhou X, Xia T, Qi H, Hu X, Shi Y. Wang X, et al. J Clin Invest. 2023 Dec 15;133(24):e163470. doi: 10.1172/JCI163470. J Clin Invest. 2023. PMID: 38099494 Free PMC article. - Enhancement of LFA-1-mediated T cell adhesion by human T lymphotropic virus type 1 p12I1.
Kim SJ, Nair AM, Fernandez S, Mathes L, Lairmore MD. Kim SJ, et al. J Immunol. 2006 May 1;176(9):5463-70. doi: 10.4049/jimmunol.176.9.5463. J Immunol. 2006. PMID: 16622014 Free PMC article. - Aggregatibacter actinomycetemcomitans Leukotoxin (LtxA; Leukothera®): Mechanisms of Action and Therapeutic Applications.
Vega BA, Belinka BA Jr, Kachlany SC. Vega BA, et al. Toxins (Basel). 2019 Aug 26;11(9):489. doi: 10.3390/toxins11090489. Toxins (Basel). 2019. PMID: 31454891 Free PMC article. Review. - Invasion of T-lymphoma cells: cooperation between Rho family GTPases and lysophospholipid receptor signaling.
Stam JC, Michiels F, van der Kammen RA, Moolenaar WH, Collard JG. Stam JC, et al. EMBO J. 1998 Jul 15;17(14):4066-74. doi: 10.1093/emboj/17.14.4066. EMBO J. 1998. PMID: 9670021 Free PMC article. - Aggregatibacter actinomycetemcomitans leukotoxin utilizes a cholesterol recognition/amino acid consensus site for membrane association.
Brown AC, Balashova NV, Epand RM, Epand RF, Bragin A, Kachlany SC, Walters MJ, Du Y, Boesze-Battaglia K, Lally ET. Brown AC, et al. J Biol Chem. 2013 Aug 9;288(32):23607-21. doi: 10.1074/jbc.M113.486654. Epub 2013 Jun 21. J Biol Chem. 2013. PMID: 23792963 Free PMC article.
References
- Berendt AR, McDowall A, Craig AG, Bates PA, Sternberg MJE, Marsh K, Newbold CI, Hogg N. The binding site on ICAM-1 for Plasmodium falciparum-infected erythrocytes overlaps, but is distinct from, the LFA-1-binding site. Cell. 1992;68:71–81. - PubMed
- Breittmayer J-P, Bernard A, Aussel C. Regulation by sphingomyelinase and Sphingosine of Ca2+signals elicited by CD3 monoclonal antibody, thapsigargin, or ionomycin in the Jurkat T cell line. J Biol Chem. 1994;269:5054–5058. - PubMed
- Brown E, Hogg N. Where the outside meets the inside: integrins as activators and targets of signal transduction cascades. Immunol Lett. 1996;54:189–193. - PubMed
- Bubb MR, Senderowicz AMJ, Sausville EA, Duncan KLK, Korn ED. Jasplakinlide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269:14869–14871. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous