Diarrheagenic Escherichia coli - PubMed (original) (raw)
Review
Diarrheagenic Escherichia coli
J P Nataro et al. Clin Microbiol Rev. 1998 Jan.
Erratum in
- Clin Microbiol Rev 1998 Apr;11(2):403
Abstract
Escherichia coli is the predominant nonpathogenic facultative flora of the human intestine. Some E. coli strains, however, have developed the ability to cause disease of the gastrointestinal, urinary, or central nervous system in even the most robust human hosts. Diarrheagenic strains of E. coli can be divided into at least six different categories with corresponding distinct pathogenic schemes. Taken together, these organisms probably represent the most common cause of pediatric diarrhea worldwide. Several distinct clinical syndromes accompany infection with diarrheagenic E. coli categories, including traveler's diarrhea (enterotoxigenic E. coli), hemorrhagic colitis and hemolytic-uremic syndrome (enterohemorrhagic E. coli), persistent diarrhea (enteroaggregative E. coli), and watery diarrhea of infants (entero-pathogenic E. coli). This review discusses the current level of understanding of the pathogenesis of the diarrheagenic E. coli strains and describes how their pathogenic schemes underlie the clinical manifestations, diagnostic approach, and epidemiologic investigation of these important pathogens.
Figures
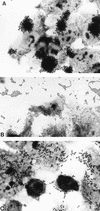
FIG. 1
The three HEp-2 adherence patterns manifested by diarrheagenic E. coli. (A) Localized adherence (LA), typical of EPEC. Bacteria form characteristic microcolonies on the surface of the HEp-2 cell. (B) Aggregative adherence (AA), which defines EAEC. Bacteria adhere to each other away from the cells as well as to the cell surface in a characteristic stacked-brick configuration. (C) Diffuse adherence (DA), which defines DAEC. Bacteria are dispersed over the surface of the cell.
FIG. 2
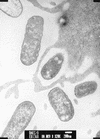
Various morphologies of diarrheagenic E. coli fimbriae as seen by transmission electron microscopy. (A) Rigid fimbrial morphology illustrated by ETEC fimbriae CS1 (labelled CFA/II in the figure). The diameter of individual fimbriae is ca. 7 nm. (B) Flexible fibrillar morphology exemplified by the CS3 component of CFA/II (arrow). Note the typical narrow diameter, ca. 2 to 3 nm, and the coiled appearance. (C) Electron micrograph showing the EPEC bundle-forming pilus expressed by strain E2348/69. Bar, 0.35 μm. Reprinted from reference with permission of the publisher.
FIG. 2
Various morphologies of diarrheagenic E. coli fimbriae as seen by transmission electron microscopy. (A) Rigid fimbrial morphology illustrated by ETEC fimbriae CS1 (labelled CFA/II in the figure). The diameter of individual fimbriae is ca. 7 nm. (B) Flexible fibrillar morphology exemplified by the CS3 component of CFA/II (arrow). Note the typical narrow diameter, ca. 2 to 3 nm, and the coiled appearance. (C) Electron micrograph showing the EPEC bundle-forming pilus expressed by strain E2348/69. Bar, 0.35 μm. Reprinted from reference with permission of the publisher.
FIG. 3
Pathogenic schemes of diarrheagenic E. coli. The six recognized categories of diarrheagenic E. coli each have unique features in their interaction with eukaryotic cells. Here, the interaction of each category with a typical target cell is schematically represented. It should be noted that these descriptions are largely the result of in vitro studies and may not completely reflect the phenomena occurring in infected humans. See the text for details.
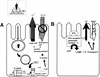
FIG. 4
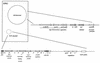
Classic mechanisms of action of ETEC toxins (see the text for details and additional proposed mechanisms). (A) LT-I. The LT holotoxin, consisting of one A subunit and five B subunits, is internalized by epithelial cells of the small bowel mucosa via endocytosis. The A1, or catalytic, subunit translocates through the vacuolar membrane and passes through the Golgi apparatus by retrograde transport. In the figure, the A subunit is shown passing through the B subunit ring, but this may not be the case in vivo. A1 catalyzes the ADP-ribosylation of arginine 201 of the α subunit of Gs-protein (which may be apically located); the ADP-ribosylated G-protein activates adenylate cyclase, which elicits supranormal levels of intracellular cAMP. cAMP is an intracellular messenger which regulates several intestinal epithelial cell membrane transporters and other host cell enzymes, as well as having effects on the cytoskeleton. The activation of the cAMP-dependent A kinase results in phosphorylation of apical membrane transporters (especially the cystic fibrosis transmembrane conductance regulator), resulting in secretion of anions (predominantly Cl− by a direct effect, and HCO3− indirectly) by crypt cells and a decrease in absorption of Na+ and Cl− by absorptive cells. cAMP may also have important effects on basolateral transporters and on intracellular calcium levels, both of which may increase the magnitude of the effects on fluid and ion transport. (B) STa. Less is known about the action of ST than of LT. ST is thought to act by binding the ST membrane receptor, GC-C. Activation of GC-C results in increased levels of intracellular cGMP. cGMP exerts its effects in increasing chloride secretion and decreasing NaCl absorption by activating the cGMP-dependent kinase (G-kinase) and/or the cAMP dependent kinase (A-kinase). Other effects of STa in inducing fluid secretion have also been postulated (see the text).
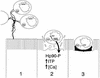
FIG. 5
Genetics of E. coli fimbriae. Genes required for the expression of functional pili are characteristically linked in gene clusters. The genetic organization of these clusters is illustrated for ETEC fimbriae CS1, CFA/I, CS3, and CS6, and for members of the Dr family, found in DAEC and EAEC. Italicized terms in parentheses represent the gene designations, to be followed by the specific letter under the corresponding arrow to the right. Arrows of similar fill pattern have genetic and functional homology; black arrows represent structural subunits. The known functions of the genes in the Dr cluster are listed below the corresponding genes. These functions can be extrapolated to arrows of similar fill pattern in the CS3 and CS6 gene cluster. The usher and chaperone genes from the Dr, CS6, and CS3 clusters have homology to the genes serving these functions in pap fimbriae: usher proteins are OMPs which serve as pores for the transport and assembly of the fimbrial shaft; fimbrial chaperones bind to the fimbrial subunit proteins in the periplasmic space and prevent premature folding and degradation. CS1 and CFA/I accessory genes, required for assembly and transport of the fimbriae, are homologous to each other but not to CS3, CS6, or the Dr family. CS6 has an unusual organization in that the first two genes of the cluster apparently encode heterologous major subunit proteins (699); the significance of this feature is not yet understood.
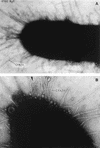
FIG. 6
Characteristic EPEC A/E lesion observed in the ileum after oral inoculation of gnotobiotic piglets. Note the intimate attachment of the bacteria to the enterocyte membrane with disruption of the apical cytoskeleton. The appearance of a bacterium sitting on a “pedestal” of cell membrane is quite characteristic. Reprinted from reference with permission of the publisher.
FIG. 7
Three-stage model of EPEC pathogenesis. (A) The first stage is characterized by initial, relatively distant interaction of bacteria with the enterocyte layer. This initial attachment is thought to be mediated by the bundle-forming pilus. (B) In the second stage, eae and other genes are activated, causing dissolution of the normal microvillar structure. (C) In the third stage, the bacterium binds closely to the epithelial membrane via the protein intimin. Other bacterial gene products mediate further disruption of the cytoskeleton and phosphorylation of cellular proteins. Modified from reference with permission of the publisher.
FIG. 8
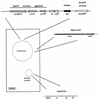
Genes involved in EPEC pathogenesis. Genes involved in the pathogenesis of EPEC-induced diarrhea are presented in schematic fashion. Chromosomal virulence genes are clustered within the LEE, which encodes a type III secretory apparatus as well as intimin and a cluster of secreted effector proteins. The EAF plasmid encodes the BFP as well as a cluster of genes required for normal expression of BFP and intimin.
FIG. 9
Genes involved in EHEC pathogenesis. Genes involved in EHEC pathogenesis are similar to those implicated for EPEC, except for the presence of the Stx-encoding phage on the EHEC chromosome and the presence of the characteristic EHEC 60-MDa plasmid instead of the EAF plasmid of EPEC. The EHEC plasmid is known to encode the enterohemolysin (ehx) as well as a fimbrial antigen potentially involved in colonization.
FIG. 10
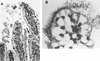
Interaction of EAEC with the intestinal epithelium. (A) Photomicrograph of the ileum of a gnotobiotic piglet fed EAEC strain 042. The arrow points to the thick mucus gel adhering to the intestinal mucosa (666). (B) High magnification of the ileal mucosa of a piglet fed EAEC strain 17-2 as in panel A. Note the aggregates of bacteria coating the villous surface. The villi are edematous, and the enterocytes themselves appear swollen. Reprinted from reference with permission of the publisher.
FIG. 11
Cytotoxicity of EAEC on T84 cells infected with EAEC strain 042. Note the aggregative adherence of bacteria to the apical membrane, associated with a loss of microvilli and the rounding of the apical membrane. Reprinted from reference with permission of the publisher.
FIG. 12
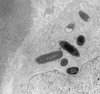
Interaction of EIEC with epithelial cells. Like Shigella, EIEC strains invade intestinal epithelial cells, lyse the phagosomal vacuole, and move through the cytoplasm, ultimately spreading to adjacent epithelial cells. This electron photomicrograph shows an EIEC organism free within the cytoplasm of an infected cell. Photo courtesy of P. Small.
FIG. 13
Genes involved in EIEC pathogenesis. Both plasmid and chromosomal genes are involved in conferring pathogenicity on EIEC strains; the genes depicted in the figure have largely been elucidated in Shigella, but most, if not all, also exist in EIEC. The chromosomal locus kcpA activates transcription of the plasmid-borne gene virG, which encodes an OMP required for directional movement through the cytoplasm. The plasmid-borne locus virK increases the surface expression of the VirG protein through an unknown mechanism. A regulatory cascade of virulence genes has been described in which the plasmid locus virF interacts with the chromosomal locus virR to regulate the transcription of the ipa gene cluster of secreted effector proteins. ipa regulation involves the intermediate regulator virB. The mxi and spa loci encode a type III secretory system homologous to that of EPEC and EHEC.
FIG. 14
Interaction of DAEC with epithelial cells. DAEC strain C1845 incubated with HEp-2 cells for 8 h. Note the association of the bacteria with the membrane and the formation of long finger-like projections emanating from the cell. These projections wrap around the bacterium in a phenotype termed “embedding.” Invasion is rarely seen. Reprinted from reference with permission of the publisher.
Similar articles
- Diagnosisand Investigation of Diarrheagenic Escherichia coli.
Nataro JP, Martinez J. Nataro JP, et al. Methods Mol Med. 1998;15:387-406. doi: 10.1385/0-89603-498-4:387. Methods Mol Med. 1998. PMID: 21390758 - Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent.
Levine MM. Levine MM. J Infect Dis. 1987 Mar;155(3):377-89. doi: 10.1093/infdis/155.3.377. J Infect Dis. 1987. PMID: 3543152 Review. - Multiplex PCR for diagnosis of enteric infections associated with diarrheagenic Escherichia coli.
Vidal R, Vidal M, Lagos R, Levine M, Prado V. Vidal R, et al. J Clin Microbiol. 2004 Apr;42(4):1787-9. doi: 10.1128/JCM.42.4.1787-1789.2004. J Clin Microbiol. 2004. PMID: 15071051 Free PMC article. - [The characteristic of diarrheagenic Escherichia separated from children aged under 5 years old in Yaroslavl.].
Kartsev NN, Svetoch EA, Ershova MG, Abrosimova GN, Tazina OI, Pinchuk AS, Fursova NK, Shepelin AP, Dyatlov IA. Kartsev NN, et al. Klin Lab Diagn. 2018;63(4):249-253. doi: 10.18821/0869-2084-63-4-249-253. Klin Lab Diagn. 2018. PMID: 30677283 Russian. - Diarrhoeagenic Escherichia coli--an emerging problem?
Clarke SC. Clarke SC. Diagn Microbiol Infect Dis. 2001 Nov;41(3):93-8. doi: 10.1016/s0732-8893(01)00303-0. Diagn Microbiol Infect Dis. 2001. PMID: 11750160 Review.
Cited by
- Etiology of Acute Diarrhea in Tunisian Children with Emphasis on Diarrheagenic Escherichia coli: Prevalence and Identification of E. coli Virulence Markers.
Ben Salem-Ben Nejma I, Hassine Zaafrane M, Hassine F, Sdiri-Loulizi K, Ben Said M, Aouni M, Mzoughi R. Ben Salem-Ben Nejma I, et al. Iran J Public Health. 2014 Jul;43(7):947-60. Iran J Public Health. 2014. PMID: 25909062 Free PMC article. - Electrochemical aptasensor for Staphylococcus aureus by stepwise signal amplification.
Zhou H, Guo W, Wang S, Hao T, Wang Z, Hu Y, Wang S, Xie J, Jiang X, Guo Z. Zhou H, et al. Mikrochim Acta. 2022 Aug 29;189(9):353. doi: 10.1007/s00604-022-05401-7. Mikrochim Acta. 2022. PMID: 36031653 - Protection against Shiga-Toxigenic Escherichia coli by Non-Genetically Modified Organism Receptor Mimic Bacterial Ghosts.
Paton AW, Chen AY, Wang H, McAllister LJ, Höggerl F, Mayr UB, Shewell LK, Jennings MP, Morona R, Lubitz W, Paton JC. Paton AW, et al. Infect Immun. 2015 Sep;83(9):3526-33. doi: 10.1128/IAI.00669-15. Epub 2015 Jun 22. Infect Immun. 2015. PMID: 26099582 Free PMC article. - Impact of Some Ecological Factors on Fecal Contamination of Drinking Water by Diarrheagenic Antibiotic-Resistant Escherichia coli in Zagazig City, Egypt.
Fakhr AE, Gohar MK, Atta AH. Fakhr AE, et al. Int J Microbiol. 2016;2016:6240703. doi: 10.1155/2016/6240703. Epub 2016 Sep 20. Int J Microbiol. 2016. PMID: 27725834 Free PMC article. - Prevalence and characterization of verotoxigenic-Escherichia coli isolates from pigs in Malaysia.
Ho WS, Tan LK, Ooi PT, Yeo CC, Thong KL. Ho WS, et al. BMC Vet Res. 2013 Jun 4;9:109. doi: 10.1186/1746-6148-9-109. BMC Vet Res. 2013. PMID: 23731465 Free PMC article.
References
- Abdul A A, Faruque S M, Ahmad Q S, Hossain K M, Mahalanabis D, Albert M J. Evaluation of a non-radioactive chemiluminescent method for using oligonucleotide and polynucleotide probes to identify enterotoxigenic Escherichia coli. J Diar Dis Res. 1994;12:113–116. - PubMed
- Acheson D W K, DeBreuker S, Donohue-Rolfe A, Kozak K, Yi A, Keusch G T. Development of a clinically useful diagnostic enzyme immunoassay for enterohemorrhagic Escherichia coli infection. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing Escherichia coli infections. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 109–112.
Publication types
MeSH terms
Grants and funding
- AI-41325/AI/NIAID NIH HHS/United States
- R01 AI033096/AI/NIAID NIH HHS/United States
- AI-33096/AI/NIAID NIH HHS/United States
- AI-21657/AI/NIAID NIH HHS/United States
- R01 AI021657/AI/NIAID NIH HHS/United States
- R37 AI021657/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical