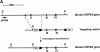The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor - PubMed (original) (raw)
The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor
S D Spencer et al. J Exp Med. 1998.
Abstract
The orphan receptor CRF2-4 is a member of the class II cytokine receptor family (CRF2), which includes the interferon receptors, the interleukin (IL) 10 receptor, and tissue factor. CRFB4, the gene encoding CRF2-4, is located within a gene cluster on human chromosome 21 that comprises three interferon receptor subunits. To elucidate the role of CRF2-4, we disrupted the CRFB4 gene in mice by means of homologous recombination. Mice lacking CRF2-4 show no overt abnormalities, grow normally, and are fertile. CRF2-4 deficient cells are normally responsive to type I and type II interferons, but lack responsiveness to IL-10. By approximately 12 wk of age, the majority of mutant mice raised in a conventional facility developed a chronic colitis and splenomegaly. Thus, CRFB4 mutant mice recapitulate the phenotype of IL-10-deficient mice. These findings suggest that CRF2-4 is essential for IL-10-mediated effects and is a subunit of the IL-10 receptor.
Figures
Figure 1
(A) The structure of the murine gene was predicted from the published human gene (17). Exons (black boxes) are numbered I–IV. The targeting vector was designed to replace exons I and II with the neomycin resistance gene. Homologous insertion of the targeting vector resulted in the introduction of an additional SpeI site (S). (B) Southern blot analysis of SpeI-digested ES cell DNA. Probe: 1.3-kb XbaI fragment derived from genomic sequences upstream of the targeting vector. (C) Northern blot of total RNA derived from wild-type or CRFB4−/− ES cells probed with a 1 kb XbaI-HindIII random labeled fragment derived from murine CRFB4 cDNA.
Figure 1
(A) The structure of the murine gene was predicted from the published human gene (17). Exons (black boxes) are numbered I–IV. The targeting vector was designed to replace exons I and II with the neomycin resistance gene. Homologous insertion of the targeting vector resulted in the introduction of an additional SpeI site (S). (B) Southern blot analysis of SpeI-digested ES cell DNA. Probe: 1.3-kb XbaI fragment derived from genomic sequences upstream of the targeting vector. (C) Northern blot of total RNA derived from wild-type or CRFB4−/− ES cells probed with a 1 kb XbaI-HindIII random labeled fragment derived from murine CRFB4 cDNA.
Figure 1
(A) The structure of the murine gene was predicted from the published human gene (17). Exons (black boxes) are numbered I–IV. The targeting vector was designed to replace exons I and II with the neomycin resistance gene. Homologous insertion of the targeting vector resulted in the introduction of an additional SpeI site (S). (B) Southern blot analysis of SpeI-digested ES cell DNA. Probe: 1.3-kb XbaI fragment derived from genomic sequences upstream of the targeting vector. (C) Northern blot of total RNA derived from wild-type or CRFB4−/− ES cells probed with a 1 kb XbaI-HindIII random labeled fragment derived from murine CRFB4 cDNA.
Figure 2
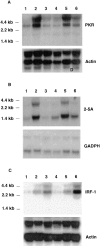
Northern blot analysis of IFN-inducible genes. Wild-type (lanes 1–3) or CRFB4−/− ES cells (lanes 4–6) were cultured under differentiating conditions and treated for 4 h at 37°C with 1,000 U/ml IFN-α2/α1 (lanes 2 and 5), 1 μg/ml muIFNγ (lanes 3 and 6; Genentech, Inc., South San Francisco, CA) or left untreated (lanes 1 and 4). Northern blot analysis was carried out with 10 μg of total RNA per lane and blots were hybridized as indicated with the probes described in Materials and Methods.
Figure 3
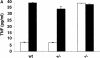
Macrophages and splenocytes derived from CRFB4−/− mice are unresponsive to IL-10. (A) TNF production in LPS-activated macrophages. Bone marrow macrophages from individual wild-type (wt), heterozygous (+/−), or homozygous mutant mice (−/−) were pretreated with 100 ng of IL-10 (white bars) or left untreated (black bars) and subsequently stimulated with 50 ng/ml of LPS before determination of TNF secretion. Indicated values are means ± SD of duplicate wells from two independent experiments. (B) 105 splenocytes or 5 × 104 peritoneal monocytes from wild-type or CRF2-4–deficient mice were treated with 10 ng of IL-10 (bold solid line) or left untreated (dotted line) for 20 h at 37°C. Cell surface expression of FcγIII/II receptor was determined by flow cytometry with anti-FcγIII/II mAb as described in Materials and Methods. The first peak of cells in the splenocyte population represents cells that do not express FcγIII/II receptors.
Figure 3
Macrophages and splenocytes derived from CRFB4−/− mice are unresponsive to IL-10. (A) TNF production in LPS-activated macrophages. Bone marrow macrophages from individual wild-type (wt), heterozygous (+/−), or homozygous mutant mice (−/−) were pretreated with 100 ng of IL-10 (white bars) or left untreated (black bars) and subsequently stimulated with 50 ng/ml of LPS before determination of TNF secretion. Indicated values are means ± SD of duplicate wells from two independent experiments. (B) 105 splenocytes or 5 × 104 peritoneal monocytes from wild-type or CRF2-4–deficient mice were treated with 10 ng of IL-10 (bold solid line) or left untreated (dotted line) for 20 h at 37°C. Cell surface expression of FcγIII/II receptor was determined by flow cytometry with anti-FcγIII/II mAb as described in Materials and Methods. The first peak of cells in the splenocyte population represents cells that do not express FcγIII/II receptors.
Figure 4
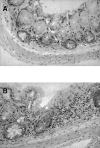
Histopathology of the large bowel from CRFB4−/− mice and control wild-type littermates. In contrast to controls showing a normal histology (A), the colon of mutant mice contains foci of colitis with mucosal inflammatory cell infiltrates composed of neutrophils, monocytes, macrophages, and lymphocytes (B). Original magnification: ×450.
References
- Morrissey JH, Fakhrai H, Edgington TS. Molecular cloning of the cDNA for tissue factor, the cellular receptor for the initiation of the coagulation protease cascade. Cell. 1987;50:129–135. - PubMed
- Harlos K, Martin D, O'Brien DP, Jones EY, Stuart DI, Polikarpov I, Miller A, Tuddenham E, Boys C. Crystal structure of the extracellular region of human tissue factor. Nature. 1994;370:662–666. - PubMed
- Liu Y, Wei SH, Ho AS, de Waal R, Malefyt, Moore KW. Expression cloning and characterization of a human IL-10 receptor. J Immunol. 1994;152:1821–1829. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials