Acquisition of Ly49 receptor expression by developing natural killer cells - PubMed (original) (raw)
Acquisition of Ly49 receptor expression by developing natural killer cells
J R Dorfman et al. J Exp Med. 1998.
Abstract
The formation of the repertoire of mouse natural killer (NK) cell receptors for major histocompatibility complex (MHC) class I molecules was investigated by determining the developmental pattern of Ly49 receptor expression. During the first days after birth, few or no splenic NK cells express Ly49A, Ly49C, Ly49G2, or Ly49I receptors. The proportion of Ly49+ splenic NK cells gradually rises to adult levels during the first 6-8 wk of life. The appearance of appreciable numbers of splenic Ly49+ NK cells coincides with the appearance of NK activity at 3-4 wk. After in vivo transfer, NK cells not expressing specific Ly49 receptors can give rise to NK cells that do, and cells expressing one of these four Ly49 receptors can give rise to cells expressing others. Once initiated, expression of a Ly49 receptor is stable for at least 10 d after in vivo transfer. Hence, initiation of Ly49 receptor expression occurs successively. Interestingly, expression of one of the receptors tested, Ly49A, did not occur after in vivo transfer of Ly49A- cells. One possible explanation for these data is that the order of Ly49 receptor expression by NK cells is nonrandom. The results provide a framework for evaluating models of NK cell repertoire formation, and how the repertoire is molded by host class I MHC molecules.
Figures
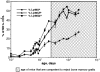
Figure 1
Ontogeny of expression of Ly49 receptors by splenic NK cells. Early splenic NK1.1+CD3− cells do not express Ly49 receptors and gradually acquire them during postnatal life. The proportion of NK1.1+CD3− cells (± SD where applicable) expressing the indicated receptor(s) is shown. The same trends were not observed when splenic NK1.1+ T (NK1.1+CD3+) cells were examined (data not shown).
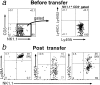
Figure 2
Ly49A−C−G2−I− NK cells express some Ly49 receptors after in vivo transfer. Ly49A−C−G2−I− NK cells were purified by cell sorting from B6-derived, NK-enriched splenocytes and then transferred into Ly5 congenic mice as described in the Materials and Methods. (a) Post-sort analysis (before transfer) from a representative experiment. (b and c) Splenocytes from an HBSS injected control (b) or a recipient (c) were stained for Ly5.2, the donor marker. (d–f) Donor-derived cells were stained for NK1.1 vs. Ly49A (d), Ly49C/I (e), or Ly49G2 ( f ). Numbers indicate the percentage of events in the corresponding quadrant except the number in parentheses, which indicates the percentage of donor NK1.1+ cells in the corresponding quadrant. Cells in the donor gate also failed to stain with antibody specific for the host-type Ly5.1 allotype (not shown).
Figure 3
Cells expressing particular Ly49 receptors before transfer continue to express them after in vivo transfer. B6 NK cells sorted for expression of the Ly49A (a), Ly49C/I (b), or Ly49G2 (c) receptors were transferred into B6-Ly5 congenic mice. Analyses of the cells before transfer (left panels) and 10 d after transfer (right panels) are shown. Note that these analyses were performed on different days on different flow cytometers; therefore, staining intensities are not directly comparable. Numbers in each quadrant indicate percentages of total events in each quadrant, and numbers in parentheses are percentages of donor-derived NK1.1+ cells. On the right side of the panels, a summary is shown of all experiments in which NK cells expressing the indicated receptor were transferred. Ly49G2 summary values include values from experiments in which Ly49G2 single positive cells were injected, while Ly49A values are derived exclusively from experiments in which Ly49A single positive cells were transferred (see Table 2). a and Fig. 4 contain data derived from a single experiment. Values are indicated as percentages of donor derived NK1.1+ cells ± SD.
Figure 4
Ly49A single positive (Ly49A+C−G2−I−) NK cells express Ly49C/I and Ly49G2 after in vivo transfer. Ly49A+C−G2−I− NK cells were purified by cell sorting from B6-derived, NK-enriched splenocytes and then transferred into Ly5 congenic mice as described in Materials and Methods. (a) Post-sort analysis (before transfer) from a representative experiment. (b) Donor-derived cells from the same experiment 10 d after transfer were stained for NK1.1 vs. Ly49A, Ly49C/I or Ly49G2. Numbers in each quadrant indicate percentages of events in each quadrant except numbers in parentheses, which are percentages of donor-derived NK1.1+ cells. Data in this figure and Fig. 3_a_ are derived from the same experiment.
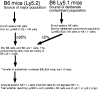
Figure 5
Experimental design to assess contribution of cellular contaminants to Ly49C/I or G2+ populations present after cell transfer. Ly49A−C−G2−I− NK1.1+ cells were purposefully contaminated with tracer Ly5 congenic cells, either Ly49C/I or G2+ NK cells or NK1.1− cells, and transferred into irradiated BALB.B recipients. The contribution of the added contaminating cells to the population 10 d after transfer was then determined.
Figure 6
NK cells do not arise from splenic NK1.1− contaminants in the starting Ly49A−C−G2−I− NK population. Cells were purified, mixed and inoculated into BALB.B mice as described in Fig. 5. NK cells of B6 and B6 Ly5.1 (marked contaminant) origin were detected by their expression of NK1.1, which is not expressed in BALB.B mice. NK cells derived from the marked contaminant NK1.1− population were detected by their coordinate expression of Ly5.1 and NK1.1. The marked contaminant cells represented 70% of NK1.1− cells in the injected population 10 d earlier (not shown). However, few or none of the NK1.1+ cells after transfer were derived from these contaminants. Additionally, none of the Ly49C/ G2/I+ NK cells were of B6 Ly5.1 origin (not shown). Numbers denote the percentages of total cells present in each quadrant. Ly5.1− cells include both host origin and B6 origin cells. The oddly shaped NK1.1− Ly5.1− population was an artifact of the flow cytometry analysis, as shown by parallel analysis of BALB.B mice inoculated with HBSS.
Similar articles
- MHC class I-Ly49 interactions shape the Ly49 repertoire on murine NK cells.
Fahlén L, Lendahl U, Sentman CL. Fahlén L, et al. J Immunol. 2001 Jun 1;166(11):6585-92. doi: 10.4049/jimmunol.166.11.6585. J Immunol. 2001. PMID: 11359811 - NK cells developing in vitro from fetal mouse progenitors express at least one member of the Ly49 family that is acquired in a time-dependent and stochastic manner independently of CD94 and NKG2.
Fraser KP, Gays F, Robinson JH, van Beneden K, Leclercq G, Vance RE, Raulet DH, Brooks CG. Fraser KP, et al. Eur J Immunol. 2002 Mar;32(3):868-78. doi: 10.1002/1521-4141(200203)32:3<868::AID-IMMU868>3.0.CO;2-A. Eur J Immunol. 2002. PMID: 11870631 - Clonal analysis of NK cell development from bone marrow progenitors in vitro: orderly acquisition of receptor gene expression.
Williams NS, Kubota A, Bennett M, Kumar V, Takei F. Williams NS, et al. Eur J Immunol. 2000 Jul;30(7):2074-82. doi: 10.1002/1521-4141(200007)30:7<2074::AID-IMMU2074>3.0.CO;2-#. Eur J Immunol. 2000. PMID: 10940897 - Specificity, tolerance and developmental regulation of natural killer cells defined by expression of class I-specific Ly49 receptors.
Raulet DH, Held W, Correa I, Dorfman JR, Wu MF, Corral L. Raulet DH, et al. Immunol Rev. 1997 Feb;155:41-52. doi: 10.1111/j.1600-065x.1997.tb00938.x. Immunol Rev. 1997. PMID: 9059881 Review. - MHC class I recognition by Ly49 natural killer cell receptors.
Natarajan K, Dimasi N, Wang J, Margulies DH, Mariuzza RA. Natarajan K, et al. Mol Immunol. 2002 May;38(14):1023-7. doi: 10.1016/s0161-5890(02)00031-7. Mol Immunol. 2002. PMID: 11955594 Review.
Cited by
- The unique neonatal NK cells: a critical component required for neonatal autoimmune disease induction by maternal autoantibody.
Rival C, Setiady Y, Samy ET, Harakal J, Tung KS. Rival C, et al. Front Immunol. 2014 May 28;5:242. doi: 10.3389/fimmu.2014.00242. eCollection 2014. Front Immunol. 2014. PMID: 24904590 Free PMC article. Review. - Prenatal tobacco smoke exposure predisposes offspring mice to exacerbated allergic airway inflammation associated with altered innate effector function.
Ferrini M, Carvalho S, Cho YH, Postma B, Miranda Marques L, Pinkerton K, Roberts K, Jaffar Z. Ferrini M, et al. Part Fibre Toxicol. 2017 Aug 22;14(1):30. doi: 10.1186/s12989-017-0212-6. Part Fibre Toxicol. 2017. PMID: 28830530 Free PMC article. - Complex expression of natural killer receptor genes in single natural killer cells.
Husain Z, Alper CA, Yunis EJ, Dubey DP. Husain Z, et al. Immunology. 2002 Jul;106(3):373-80. doi: 10.1046/j.1365-2567.2002.01444.x. Immunology. 2002. PMID: 12100725 Free PMC article. - NK cells in allogeneic bone marrow transplantation.
Voutsadakis IA. Voutsadakis IA. Cancer Immunol Immunother. 2003 Sep;52(9):525-34. doi: 10.1007/s00262-003-0378-7. Cancer Immunol Immunother. 2003. PMID: 14627124 Free PMC article. Review. - NK/ILC1 cells mediate neuroinflammation and brain pathology following congenital CMV infection.
Kveštak D, Juranić Lisnić V, Lisnić B, Tomac J, Golemac M, Brizić I, Indenbirken D, Cokarić Brdovčak M, Bernardini G, Krstanović F, Rožmanić C, Grundhoff A, Krmpotić A, Britt WJ, Jonjić S. Kveštak D, et al. J Exp Med. 2021 May 3;218(5):e20201503. doi: 10.1084/jem.20201503. J Exp Med. 2021. PMID: 33630019 Free PMC article.
References
- Ljunggren HG, Kärre K. In search of the ‘missing self ': MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. - PubMed
- Yokoyama WM, Seaman WE. The Ly-49 and NKR-P1 gene families encoding lectin-like receptors on natural killer cells: the NK gene complex. Annu Rev Immunol. 1993;11:613–635. - PubMed
- Held W, Dorfman JR, Wu M-F, Raulet DH. Major histocompatibility complex class I-dependent skewing of the natural killer cell Ly49 receptor repertoire. Eur J Immunol. 1996;26:2286–2292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials