Modulation of intracellular transport by transported proteins: insight from regulation of COPI-mediated transport - PubMed (original) (raw)
Modulation of intracellular transport by transported proteins: insight from regulation of COPI-mediated transport
T Aoe et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Intracellular transport is best understood for how proteins are shuttled among different compartments of the secretory pathway by membrane-bound transport carriers. However, it remains unclear whether regulation of this transport is modulated by the transported (cargo) proteins in the lumen of transport pathways. In the early secretory pathways that connect the endoplasmic reticulum (ER) and the Golgi complex, the small GTPase ADP-ribosylation factor 1 (ARF1) recruits a cytosolic coat protein complex named COPI onto membranes as a key step in the formation of transport vesicles. Transport of newly synthesized proteins that leave the ER includes a class of cargo proteins with a sequence motif of KDEL. When these KDEL proteins leave the ER to reach the Golgi complex, they are recognized by their receptor and transported retrograde in COPI-coated vesicles back to the ER. We now demonstrate that stimulation of the KDEL receptor by a KDEL protein enhances an interaction between the KDEL receptor and a GTPase-activating protein for ARF1. As a result, more cytosolic GTPase-activating protein is recruited to membranes to inactivate ARF1. Thus, the KDEL proteins are examples of luminal cargo proteins that regulate transport by activating their receptor. Most likely, this regulation affects retrograde transport from the Golgi complex to the ER, as activated KDEL receptor appears to reside only in retrograde COPI-coated vesicles.
Figures
Figure 1
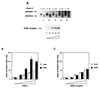
KDEL proteins modulate the association of the KDEL receptor with GAP. (A) The association between the KDEL receptor and GAP is enhanced by the overexpression of lysozyme-KDEL. A HeLa cell line that stably expresses myc-tagged KDEL receptor was either induced or uninduced for lysozyme-KDEL overexpression, and then lysed and immunoprecipitated with an anti-myc antibody followed by immunoblotting with an anti-ARF1 GAP antiserum (Top) or an anti-myc antibody (Middle). The same cell lysate also was immunoprecipitated with an anti-lysozyme antibody followed by Western blotting for lysozyme to assess the level of lysozyme-KDEL expressed (Bottom). (B) The association of KDEL receptor and GAP requires the specific recognition of the KDEL sequence by the receptor. A HeLa cell line that stably expresses both HA-tagged wild-type receptor and myc-tagged mutant receptor was either induced or uninduced for lysozyme-KDEL overexpression, and then immunoprecipitated with either an anti-myc antibody (Left) or an anti-HA antibody (Right) followed by immunoblotting with either an anti-ARF1 GAP antiserum (Top) or the appropriate anti-epitope tag antibody (Middle). The same cell lysate also was immunoprecipitated with an anti-lysozyme antibody followed by Western blotting for lysozyme to assess the level of lysozyme-KDEL expressed (Bottom).
Figure 2
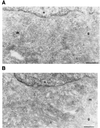
Overexpression of lysozyme-KDEL increases GAP recruitment to membranes of the early secretory system. A HeLa cell line with inducible expression of lysozyme-KDEL was either uninduced (A) or induced (B), and then examined by immunogold electron microscopy using an anti-ARF1 GAP antiserum (10 nm gold) (bar, 200 nm). g, Golgi; m, mitochondrion; n, nucleus. Quantitation of enhanced GAP recruitment upon induction of lysozyme-KDEL is shown in Table 1.
Figure 3
KDEL proteins modulate the ability of the KDEL receptor to induce the phenotype of ARF1 inactivation, as assessed by Golgi redistribution to the ER. (A) Increasing receptor expression increases Golgi redistribution to the ER. HeLa cells were transiently transfected with increasing amounts of a plasmid encoding the KDEL receptor, and then assessed for Golgi-specific glycosylation of Tac-E19 (Upper). Direct immunoblotting of whole-cell lysates with an anti-myc antibody reveals increasing level of receptor expression (Lower). (B) The co-overexpression of lysozyme-KDEL and the KDEL receptor enhances the ability of the receptor to induce Golgi redistribution to the ER. HeLa cells were transiently transfected with either nothing or a plasmid encoding lysozyme-KDEL and increasing amounts of a plasmid encoding the KDEL receptor, and then assessed for Golgi-specific glycosylation of Tac-E19. The ratio of endo H-resistant to endo H-sensitive Tac-E19 is quantified for three separate experiments, and the bar graph represents the mean with standard error. There is a significant difference (P < 10−4) in the effect of co-overexpressing the KDEL receptor with its KDEL ligand (KDEL) versus overexpressing the receptor alone (None), by the two-way factorial ANOVA and contrast test. (C) The co-overexpression of lysozyme-AARL and the KDEL receptor does not enhance the ability of the receptor to induce Golgi redistribution to the ER. HeLa cells were transiently transfected with either nothing or a plasmid encoding lysozyme-AARL and increasing amounts of a plasmid encoding the KDEL receptor, and then assessed for Golgi-specific glycosylation of Tac-E19. The results of three separate experiments are quantified, and their mean with standard error are represented by a bar graph. There is no significant difference (P = 0.96) in comparing the effects of overexpressing the KDEL receptor with a control ligand (AARL) versus overexpressing the receptor alone (None), by the two-way factorial ANOVA and contrast test.
Similar articles
- Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport.
Aridor M, Bannykh SI, Rowe T, Balch WE. Aridor M, et al. J Cell Biol. 1995 Nov;131(4):875-93. doi: 10.1083/jcb.131.4.875. J Cell Biol. 1995. PMID: 7490291 Free PMC article. - COPII vesicles derived from mammalian endoplasmic reticulum microsomes recruit COPI.
Rowe T, Aridor M, McCaffery JM, Plutner H, Nuoffer C, Balch WE. Rowe T, et al. J Cell Biol. 1996 Nov;135(4):895-911. doi: 10.1083/jcb.135.4.895. J Cell Biol. 1996. PMID: 8922375 Free PMC article. - The retrieval function of the KDEL receptor requires PKA phosphorylation of its C-terminus.
Cabrera M, Muñiz M, Hidalgo J, Vega L, Martín ME, Velasco A. Cabrera M, et al. Mol Biol Cell. 2003 Oct;14(10):4114-25. doi: 10.1091/mbc.e03-04-0194. Epub 2003 Aug 7. Mol Biol Cell. 2003. PMID: 14517323 Free PMC article. - Building a secretory apparatus: role of ARF1/COPI in Golgi biogenesis and maintenance.
Lippincott-Schwartz J, Cole NB, Donaldson JG. Lippincott-Schwartz J, et al. Histochem Cell Biol. 1998 May-Jun;109(5-6):449-62. doi: 10.1007/s004180050247. Histochem Cell Biol. 1998. PMID: 9681627 Review. - Biosynthetic protein transport through the early secretory pathway.
Nickel W, Wieland FT. Nickel W, et al. Histochem Cell Biol. 1998 May-Jun;109(5-6):477-86. doi: 10.1007/s004180050249. Histochem Cell Biol. 1998. PMID: 9681629 Review.
Cited by
- Dilated cardiomyopathy caused by aberrant endoplasmic reticulum quality control in mutant KDEL receptor transgenic mice.
Hamada H, Suzuki M, Yuasa S, Mimura N, Shinozuka N, Takada Y, Suzuki M, Nishino T, Nakaya H, Koseki H, Aoe T. Hamada H, et al. Mol Cell Biol. 2004 Sep;24(18):8007-17. doi: 10.1128/MCB.24.18.8007-8017.2004. Mol Cell Biol. 2004. PMID: 15340063 Free PMC article. - The KDEL receptor mediates a retrieval mechanism that contributes to quality control at the endoplasmic reticulum.
Yamamoto K, Fujii R, Toyofuku Y, Saito T, Koseki H, Hsu VW, Aoe T. Yamamoto K, et al. EMBO J. 2001 Jun 15;20(12):3082-91. doi: 10.1093/emboj/20.12.3082. EMBO J. 2001. PMID: 11406585 Free PMC article. - The Role of BiP Retrieval by the KDEL Receptor in the Early Secretory Pathway and its Effect on Protein Quality Control and Neurodegeneration.
Jin H, Komita M, Aoe T. Jin H, et al. Front Mol Neurosci. 2017 Jul 17;10:222. doi: 10.3389/fnmol.2017.00222. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28769758 Free PMC article. Review. - Aminoacyl-tRNA synthetase-interacting multifunctional protein 1/p43 controls endoplasmic reticulum retention of heat shock protein gp96: its pathological implications in lupus-like autoimmune diseases.
Han JM, Park SG, Liu B, Park BJ, Kim JY, Jin CH, Song YW, Li Z, Kim S. Han JM, et al. Am J Pathol. 2007 Jun;170(6):2042-54. doi: 10.2353/ajpath.2007.061266. Am J Pathol. 2007. PMID: 17525271 Free PMC article. - ARF-GAP-mediated interaction between the ER-Golgi v-SNAREs and the COPI coat.
Rein U, Andag U, Duden R, Schmitt HD, Spang A. Rein U, et al. J Cell Biol. 2002 Apr 29;157(3):395-404. doi: 10.1083/jcb.200112092. Epub 2002 Apr 22. J Cell Biol. 2002. PMID: 11970962 Free PMC article.
References
- Peyroche A, Paris S, Jackson C L. Nature (London) 1996;384:479–481. - PubMed
- Chardin P, Paris S, Antonny B, Robineau S, Beraud-Dufour S, Jackson C L, Chabre M. Nature (London) 1996;384:481–484. - PubMed
- Serafini T, Orci L, Amherdt M, Brunner M, Kahn R A, Rothman J E. Cell. 1991;67:239–253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials