Role of cyclophilin A in the uptake of HIV-1 by macrophages and T lymphocytes - PubMed (original) (raw)
Role of cyclophilin A in the uptake of HIV-1 by macrophages and T lymphocytes
B Sherry et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Cyclophilins are a family of proteins that bind cyclosporin A (CsA) and possess peptidyl-prolyl cis-trans isomerase activity. In addition, they are secreted by activated cells and act in a cytokine-like manner, presumably via signaling through a cell surface cyclophilin receptor. More recently, host-derived cyclophilin A (CyPA) has been shown to be incorporated into HIV-1 virions and its incorporation essential for viral infectivity. Here we present evidence supporting a role for viral-associated CyPA in the early events of HIV-1 infection. We report that HIV-1 infection of primary peripheral blood mononuclear cells can be inhibited by: (i) an excess of exogenously added CyPA; (ii) a CsA analogue unable to enter the cells; (iii) neutralizing antibodies to CyPA. Taken together with our observations that recombinant CyPA-induced mobilization of calcium in immortalized, as well as primary, CD4+ T lymphocytes, and that incubation of T cells with iodinated CyPA, followed by chemical cross-linking, resulted in the formation of a high molecular mass complex on the cell surface, these results suggest that HIV-1-associated CyPA mediates an early event in viral infection via interaction with a cellular receptor. This interaction may present a target for anti-HIV therapies and vaccines.
Figures
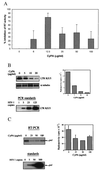
Figure 1
Recombinant CyPA inhibits HIV-1 infection. (A) PBMCs from 6 different donors were infected with HIV-1RF in the presence of indicated concentrations of recombinant murine CyPA. At the peak of viral infection (typically day 7 to 11 after infection), RT activity was measured in culture supernatants. The results were tabulated as percentage of inhibition relative to untreated HIV-infected cultures from the same donor. The data are mean ± SEM across donors. (B) PBMCs were infected with HIV-1RF in the presence of indicated concentrations of recombinant murine CyPA. Two hours after infection, cells were washed and viral DNA was analyzed by PCR by using primers that amplify the R/U5 region of the LTR. Amplified fragments were revealed by Southern hybridization, and the amount of product was quantified on Packard Direct Imager (bar graph on the right). A fragment of the α tubulin gene was amplified from the same samples to control for possible differences in the amount of total DNA. Dilutions of 8E5 cells containing the indicated number of HIV-1 genomes were amplified in parallel as standards. Results are shown for one representative experiment out of two performed. (C) Primary differentiated human macrophages were infected with HIV-1ADA in the presence of the indicated concentrations of CyPA. Two hours after infection, cells were washed and treated with trypsin. Viral RNA then was analyzed by RT-PCR by using primers specific for the pol gene. Results of one out of two independent experiments are shown on the left. Results of both experiments were quantified on a Packard Direct Imager and plotted on the right as mean ± SE. Dilutions of 8E5 cells were amplified in parallel, as above, to provide a standard curve.
Figure 2
Cyclophilin-binding sites on CD4+ T lymphocytes. (A) Iodinated CyPA was added to H9 cells in the presence or absence of a 100-fold excess of cold CyPA. After cross-linking with a thiol-cleavable reagent, dithiobis(succinimidylpropionate), cells were extracted with Triton X-100 to solubilize membrane proteins, and the extract was analyzed by PAGE (10–15%) under either reducing (Left) or nonreducing (Right) conditions. Note that the high molecular mass complex did not migrate out of the stacking gel. (B) Analysis of intracellular calcium was performed on H9 cells (Left graph) or on purified CD4+ T cells, either quiescent or activated with phytohemagglutinin and IL-2 (Right graph).
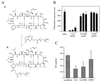
Figure 3
Extracellular CsA inhibits HIV-1 infection. (A) Synthesis of polyethyleneglycol-modified CsA (b) from 8-amino-CsA (a), an analogue of CsA that retains its immunosuppressive activity and ability to inhibit cis-trans isomerase activity of CyPA (19). (B) Incorporation of [3H]thymidine into T cells was measured in the presence of indicated concentrations of CsA, CsA-PEG, or X-PEG (control for CsA-PEG). (C) PBMCs were infected with HIV-1RF in the presence of 1 μg/ml of CsA, CsA-PEG, or X-PEG. Five days after infection, RT in the supernatant was measured. Results are mean ± SD of three independent infections with the cells from the same donor.
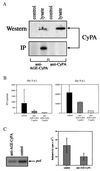
Figure 4
Antibodies to CyPA inhibit HIV-1 infection. (A). Sera from rabbits immunized with CyPA (anti-CyPA) or with AGE-modified CyPA (anti-AGE-CyPA) was used to reveal CyPA in a Western blot or immunoprecipitation (IP) assay. For Western analysis, a lysate of murine RAW 264.7 cells was fractionated on an SDS/PAGE. For IP analysis, lysate of RAW 264.7 cells metabolically labeled with [35S]methionine was immunoprecipitated with anti-CyPA or anti-AGE-CyPA serum (diluted 1:100) and fractionated on SDS/PAGE. (B) PBMCs were infected with HIV-1RF in the absence (control) or presence of affinity-purified anti-AGE-CyPA Ig. RT was measured in culture supernatants at days 6 and 8 after infection. Results are mean ± SE of three independent infections. (C) Primary human macrophages were infected with HIV-1ADA in the presence of anti-AGE-CyPA serum (1:40 dilution) or pre-immune (control) rabbit serum. Two hours after infection, cells were washed, treated with trypsin, and viral RNA was analyzed by RT-PCR by using _pol_-specific primers. Results of one out of two independent experiments are shown on the left, and both experiments are quantified on the Packard Direct Imager and plotted on the right as mean ± SE.
Similar articles
- Transfer of the HIV-1 cyclophilin-binding site to simian immunodeficiency virus from Macaca mulatta can confer both cyclosporin sensitivity and cyclosporin dependence.
Bukovsky AA, Weimann A, Accola MA, Göttlinger HG. Bukovsky AA, et al. Proc Natl Acad Sci U S A. 1997 Sep 30;94(20):10943-8. doi: 10.1073/pnas.94.20.10943. Proc Natl Acad Sci U S A. 1997. PMID: 9380739 Free PMC article. - Mechanistic independence of Nef and cyclophilin A enhancement of human immunodeficiency virus type 1 infectivity.
Aiken C. Aiken C. Virology. 1998 Aug 15;248(1):139-47. doi: 10.1006/viro.1998.9254. Virology. 1998. PMID: 9705263 Free PMC article. - Host cyclophilin A mediates HIV-1 attachment to target cells via heparans.
Saphire AC, Bobardt MD, Gallay PA. Saphire AC, et al. EMBO J. 1999 Dec 1;18(23):6771-85. doi: 10.1093/emboj/18.23.6771. EMBO J. 1999. PMID: 10581250 Free PMC article. - Human immunodeficiency virus type 1 hijacks host cyclophilin A for its attachment to target cells.
Saphire AC, Bobardt MD, Gallay PA. Saphire AC, et al. Immunol Res. 2000;21(2-3):211-7. doi: 10.1385/IR:21:2-3:211. Immunol Res. 2000. PMID: 10852119 Review. - Insights into the roles of cyclophilin A during influenza virus infection.
Liu X, Zhao Z, Liu W. Liu X, et al. Viruses. 2013 Jan 15;5(1):182-91. doi: 10.3390/v5010182. Viruses. 2013. PMID: 23322171 Free PMC article. Review.
Cited by
- Cyclophilin A-independent replication of a human immunodeficiency virus type 1 isolate carrying a small portion of the simian immunodeficiency virus SIV(MAC) gag capsid region.
Fujita M, Yoshida A, Miyaura M, Sakurai A, Akari H, Koyama AH, Adachi A. Fujita M, et al. J Virol. 2001 Nov;75(21):10527-31. doi: 10.1128/JVI.75.21.10527-10531.2001. J Virol. 2001. PMID: 11581426 Free PMC article. - Roles of cyclophilins in cancers and other organ systems.
Yao Q, Li M, Yang H, Chai H, Fisher W, Chen C. Yao Q, et al. World J Surg. 2005 Mar;29(3):276-80. doi: 10.1007/s00268-004-7812-7. World J Surg. 2005. PMID: 15706440 Review. - CD147 stimulates HIV-1 infection in a signal-independent fashion.
Pushkarsky T, Yurchenko V, Laborico A, Bukrinsky M. Pushkarsky T, et al. Biochem Biophys Res Commun. 2007 Nov 23;363(3):495-9. doi: 10.1016/j.bbrc.2007.08.192. Epub 2007 Sep 14. Biochem Biophys Res Commun. 2007. PMID: 17888876 Free PMC article. - Conformational plasticity of an enzyme during catalysis: intricate coupling between cyclophilin A dynamics and substrate turnover.
McGowan LC, Hamelberg D. McGowan LC, et al. Biophys J. 2013 Jan 8;104(1):216-26. doi: 10.1016/j.bpj.2012.11.3815. Epub 2013 Jan 8. Biophys J. 2013. PMID: 23332074 Free PMC article.
References
- Fischer G, Wittmann-Liebold B, Lang K, Kiefhaber T, Schmid F X. Nature (London) 1989;337:476–478. - PubMed
- Zhao Y, Ke H. Biochemistry. 1996;35:7356–7361. - PubMed
- Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh C T, Sodroski J, Gottlinger H G. Nature (London) 1994;372:363–365. - PubMed
- Franke E K, Yuan H E, Luban J. Nature (London) 1994;372:359–362. - PubMed
- Ott D E, Coren L V, Johnson D G, Sowder R C, Arthur L O, Henderson L E. AIDS Res Hum Retroviruses. 1995;11:1003–1006. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials