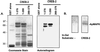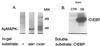Repeated pulses of serotonin required for long-term facilitation activate mitogen-activated protein kinase in sensory neurons of Aplysia - PubMed (original) (raw)
Repeated pulses of serotonin required for long-term facilitation activate mitogen-activated protein kinase in sensory neurons of Aplysia
D Michael et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Long-term facilitation of the connections between the sensory and motor neurons of the gill-withdrawal reflex in Aplysia requires five repeated pulses of serotonin (5-HT). The repeated pulses of 5-HT initiate a cascade of gene activation that leads ultimately to the growth of new synaptic connections. Several genes in this process have been identified, including the transcriptional regulators apCREB-1, apCREB-2, apC/EBP, and the cell adhesion molecule apCAM, which is thought to be involved in the formation of new synaptic connections. Here we report that the transcriptional regulators apCREB-2 and apC/EBP, as well as a peptide derived from the cytoplasmic domain of apCAM, are phosphorylated in vitro by Aplysia mitogen-activated protein kinase (apMAPK). We have cloned the cDNA encoding apMAPK and show that apMAPK activity is increased in sensory neurons treated with repeated pulses of 5-HT and by the cAMP pathway. These results suggest that apMAPK may participate with cAMP-dependent protein kinase during long-term facilitation in sensory cells by modifying some of the key elements involved in the consolidation of short- to long-lasting changes in synaptic strength.
Figures
Figure 1
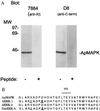
Immunoreactivity and cloning of apMAPK. (A) Antibody 7884 against rat MAPK (24) was affinity purified and used in immoblots of Aplysia central nervous system (CNS) extracts. The antibody specifically recognized a protein of 43 kDa. A λZAP II (Stratagene) expression library containing cDNAs from Aplysia total CNS was screened with the 7884 antibody. The sequence of an isolated cDNA clone was found to be 85% identical to human ERK2 and we called it apMAPK (GenBank accession no. U40484). (B) The sequence of the activation domain of apMAPK (domain VIII) is compared with the human and Drosophila homologs. The D8 antibody was made against the C-terminal 18 amino acids of the molecule, and it specifically recognized a 43-kDa protein in the Western blot analysis shown in A.
Figure 2
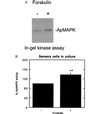
Repeated application of 5-HT onto sensory cells in culture activates apMAPK. Protein extracts from approximately 50 cells in culture were subjected to a kinase assay in MBP-containing gels (A–C, except where otherwise indicated). (A) An extract of Aplysia sensory cells in buffer A and 0.3 mg/ml BSA was treated with 1 μl of the pure MAPK phosphatase 1 (MKP-1) prior to the kinase assay in MBP-containing gels. (B) Five pulses of 10 μM 5-HT were given to sensory cells for 5 min, each pulse at 20-min intervals, while control cultures were given regular medium at the same intervals. An in-gel kinase assay was subsequently performed. The kinase activities at 58 and 63 kDa were used as references to correct and normalize for the apMAPK activities. Normalized MAPK activity as detected by the in-gel kinase assay was 64% higher in cultures treated with five pulses of 5-HT compared with control (mock treated) cultures. Also, similar experiments were performed with one pulse of 5-HT that lasted 5 min. The mean apMAPK activity after one pulse compared with control cultures was 2.7 ± 10.8%; P < 0.01 vs. five pulses of 5-HT. (C) Sensory cells in culture were treated with 30 μM PD098059 for 30 min or with vehicle (0.1% dimethyl sulfoxide) and apMAPK activity was determined by kinase analysis in MBP-containing gels. Shown is mean percentage ±SE.
Figure 3
Forskolin increases MAPK activity in pleural-pedal ganglia and in isolated cells in culture. (A) Paired pleural-pedal ganglia were treated with either 150 mM forskolin (+) or its inactive analog 1,9-dideoxyforskolin (−), for 3 hr, and a kinase assay was performed in MBP-containing gels by using fractionated apMAPK (fraction F7). (B) A similar kinase assay was performed by using sensory cells in culture that were treated with forskolin (100 μM) for 10 min (n = 2), 30 min (n = 1), or 1 hr (n = 1). Control cultures were treated with 100 μM 1,9-dideoxyforskolin. Shown is the mean percentage ±SE.
Figure 4
Phosphorylation of CREB-2 by MAPK. (A) Purified activated rat ERK2 was incubated with various CREB-2 fusion proteins, including GST alone, full-length GST-CREB-2-(1–378), and two mutants encoding the N-terminal sequence (1–288) or the C-terminal sequence (260–378) of CREB-2. The relative amounts of protein used are shown by Coomassie stain in Left. Purified active rat ERK2 was allowed to phosphorylate for 30 min. Subsequently, substrates were separated by gel electrophoresis; relative phosphorylation efficiencies are depicted in Right (2.5-hr exposure). Quantitation revealed that CREB-2 (1–378) and its low molecular weight derivatives contained 0.015 pmol of phosphate per pmol of substrate, and that CREB-2-(1–288) and its derivative contained 0.016 pmol/pmol. (B) In-gel kinase assays using no substrate, or CREB-2 as a substrate and Aplysia CNS-derived fraction F7 as the source of kinase activities. The 43-kDa kinase activity (apMAPK) was capable of phosphorylating CREB-2.
Figure 5
Aplysia C/EBP is a MAPK substrate. (A) To identify Aplysia kinase(s) that phosphorylate C/EBP, an in-gel kinase assay was performed with a partially purified kinase-containing fraction (fraction F7). The 43-kDa apMAPK activity was directed against C/EBP and MBP. In addition, a 50-kDa kinase activity was directed only against C/EBP. (B) Immune-complex kinase assays were performed with antibodies against apMAPK or control nonimmune serum. The immunoprecipitated material from extracts of isolated ganglia was incubated in each case with pure GST-C/EBP under conditions that favor MAPK activity, followed by gel electrophoresis. The autoradiogram demonstrates phosphorylation of C/EBP by immunoprecipitated apMAPK.
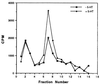
Figure 6
Effect of 5-HT on the phosphorylation of an apCAM-derived peptide by fractionated MAPK. Kinase activity against apCAM peptides was determined in control extracts or extracts from 5-HT-treated ganglia. Paired pleural-pedal ganglia were incubated in the absence (− 5-HT) or presence of 20 μM 5-HT for 30 min and cell extracts were subjected to fractionation on anion-exchange FFQ minicolumns. Samples from the individual fractions were allowed to phosphorylate apCAM-derived peptide. This experiment was repeated (n = 3) and quantitation (mean percentage change in fractions F7 and fraction F8, combined) revealed that 5-HT caused an increase in kinase activity against apCAM by 73% ± 4% (SE) (P < 0.01).
Similar articles
- Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change.
Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Bartsch D, et al. Cell. 1995 Dec 15;83(6):979-92. doi: 10.1016/0092-8674(95)90213-9. Cell. 1995. PMID: 8521521 - Overexpression of and RNA interference with the CCAAT enhancer-binding protein on long-term facilitation of Aplysia sensory to motor synapses.
Lee JA, Kim HK, Kim KH, Han JH, Lee YS, Lim CS, Chang DJ, Kubo T, Kaang BK. Lee JA, et al. Learn Mem. 2001 Jul-Aug;8(4):220-6. doi: 10.1101/lm.40201. Learn Mem. 2001. PMID: 11533225 Free PMC article. - Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation.
Dash PK, Hochner B, Kandel ER. Dash PK, et al. Nature. 1990 Jun 21;345(6277):718-21. doi: 10.1038/345718a0. Nature. 1990. PMID: 2141668 - Transcriptional regulation of long-term memory in the marine snail Aplysia.
Lee YS, Bailey CH, Kandel ER, Kaang BK. Lee YS, et al. Mol Brain. 2008 Jun 17;1:3. doi: 10.1186/1756-6606-1-3. Mol Brain. 2008. PMID: 18803855 Free PMC article. Review. - Cellular, molecular, and epigenetic mechanisms in non-associative conditioning: implications for pain and memory.
Rahn EJ, Guzman-Karlsson MC, David Sweatt J. Rahn EJ, et al. Neurobiol Learn Mem. 2013 Oct;105:133-50. doi: 10.1016/j.nlm.2013.06.008. Epub 2013 Jun 22. Neurobiol Learn Mem. 2013. PMID: 23796633 Free PMC article. Review.
Cited by
- Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus.
Lu YF, Kandel ER, Hawkins RD. Lu YF, et al. J Neurosci. 1999 Dec 1;19(23):10250-61. doi: 10.1523/JNEUROSCI.19-23-10250.1999. J Neurosci. 1999. PMID: 10575022 Free PMC article. - Protein kinase C regulates local synthesis and secretion of a neuropeptide required for activity-dependent long-term synaptic plasticity.
Hu JY, Chen Y, Schacher S. Hu JY, et al. J Neurosci. 2007 Aug 15;27(33):8927-39. doi: 10.1523/JNEUROSCI.2322-07.2007. J Neurosci. 2007. PMID: 17699674 Free PMC article. - Multiple serotonergic mechanisms contributing to sensitization in aplysia: evidence of diverse serotonin receptor subtypes.
Barbas D, DesGroseillers L, Castellucci VF, Carew TJ, Marinesco S. Barbas D, et al. Learn Mem. 2003 Sep-Oct;10(5):373-86. doi: 10.1101/lm.66103. Learn Mem. 2003. PMID: 14557610 Free PMC article. Review. - A role for ERK MAP kinase in physiologic temporal integration in hippocampal area CA1.
Selcher JC, Weeber EJ, Christian J, Nekrasova T, Landreth GE, Sweatt JD. Selcher JC, et al. Learn Mem. 2003 Jan-Feb;10(1):26-39. doi: 10.1101/lm.51103. Learn Mem. 2003. PMID: 12551961 Free PMC article. - Serotonin Receptor Agonist 5-Nonyloxytryptamine Alters the Kinetics of Reovirus Cell Entry.
Mainou BA, Ashbrook AW, Smith EC, Dorset DC, Denison MR, Dermody TS. Mainou BA, et al. J Virol. 2015 Sep;89(17):8701-12. doi: 10.1128/JVI.00739-15. Epub 2015 Jun 24. J Virol. 2015. PMID: 26109733 Free PMC article.
References
- Davis H P, Squire L R. Psychol Bull. 1984;96:518–559. - PubMed
- Goelet P, Castellucci V F, Schacher S, Kandel E R. Nature (London) 1986;322:419–422. - PubMed
- Dezazzo J, Tully T. Trends Neurosci. 1995;18:212–218. - PubMed
- Alberini C M, Ghirardi M, Huang Y-Y, Nguyen P, Kandel E R. Ann NY Acad Sci. 1995;758:261–286. - PubMed
- Carew T J. Neuron. 1996;16:5–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources